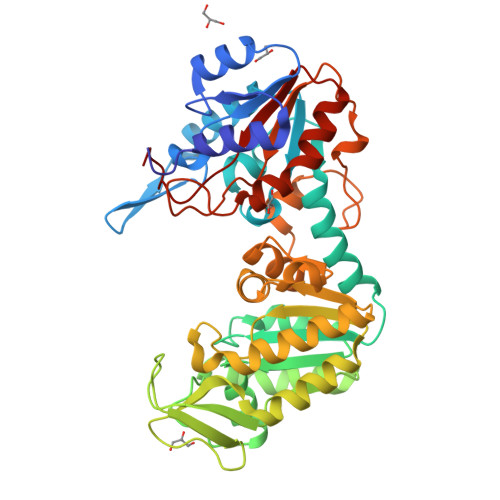
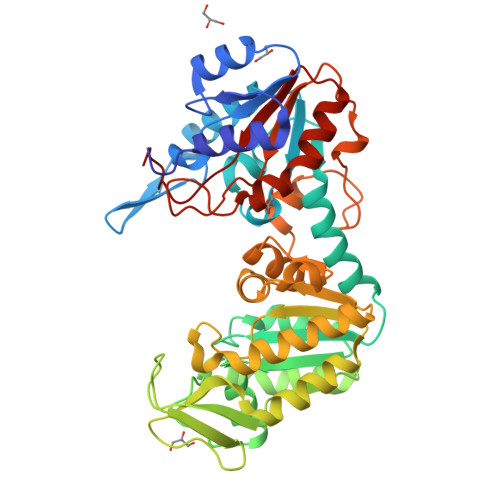
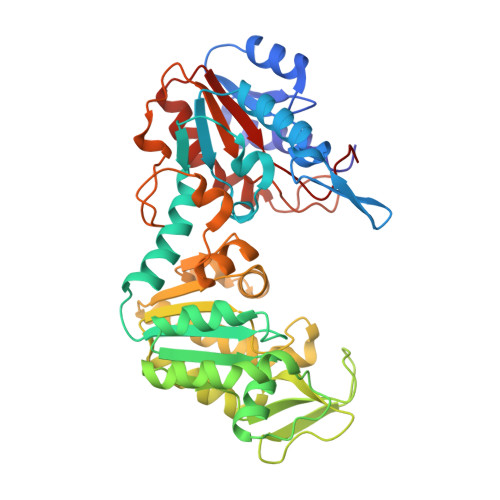
Structure of a circularly permuted phosphoglycerate kinase.
Tougard, P., Bizebard, T., Ritco-Vonsovici, M., Minard, P., Desmadril, M.(2002) Acta Crystallogr D Biol Crystallogr 58: 2018-2023
- PubMed: 12454459
- DOI: https://doi.org/10.1107/s0907444902015548
- Primary Citation of Related Structures:
1FW8 - PubMed Abstract:
The crystallographic structure of a circularly permuted form of yeast PGK, 72p yPGK, has been determined to a resolution of 2.3 A by molecular replacement. In this engineered protein, the C- and N-terminal residues of the wild-type protein are directly connected by a peptide bond and new N- and C-terminal residues are located within the N-terminal domain. The overall fold of the protein is very similar to that of the wild-type protein, directly demonstrating that the continuity of a folding unit is not relevant to the folding process of the whole protein. Only limited structural changes were observed: these were in the regions associated with the new connection, in a long flexible loop in the permuted domain and in the vicinity of Arg38, a functionally important residue. The relative positions of the two domains suggested that this permuted protein adopts one of the most open/twisted conformations seen amongst PGKs of known structure. The effect of the mutation on the functional properties is more easily accounted for by a restriction of hinge-bending motion than by structural changes in the protein.
Organizational Affiliation:
Laboratoire de Modélisation et d'Ingénierie des Protéines, UMR 8619, Université de Paris-Sud, Bâtiment 430, F-91405 Orsay CEDEX, France.