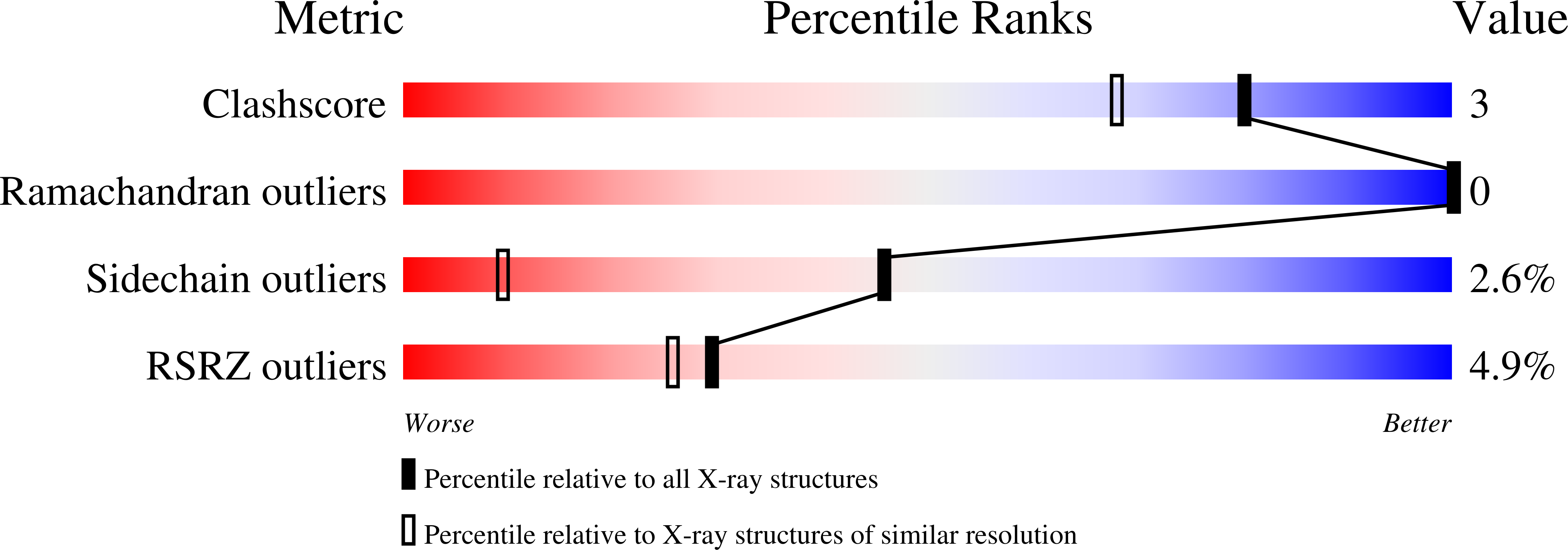
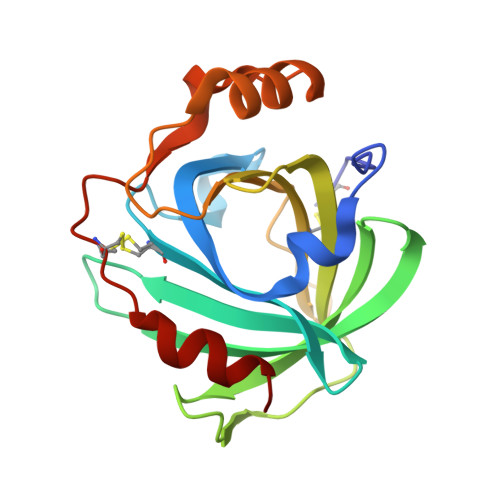
Ligand-induced heme ruffling and bent no geometry in ultra-high-resolution structures of nitrophorin 4.
Roberts, S.A., Weichsel, A., Qiu, Y., Shelnutt, J.A., Walker, F.A., Montfort, W.R.(2001) Biochemistry 40: 11327-11337
- PubMed: 11560480
- DOI: https://doi.org/10.1021/bi0109257
- PubMed Abstract:
The nitrophorins are a family of proteins that use ferric heme to transport nitric oxide (NO) from the salivary glands of blood-sucking insects to their victims, resulting in vasodilation and reduced blood coagulation. We have refined atomic resolution structures of nitrophorin 4 (NP4) from Rhodnius prolixus complexed with NO (1.08 A) and NH(3) (1.15 A), yielding a highly detailed picture of the iron coordination sphere. In NP4-NO, the NO nitrogen is coordinated to iron (Fe-N distance = 1.66 A) and is somewhat bent (Fe-N-O angle = 156 degrees ), with bending occurring in the same plane as the proximal histidine ring. The Fe(NO)(heme)(His) coordination geometry is unusual but consistent with an Fe(III) oxidation state that is stabilized by a highly ruffled heme. Heme ruffling occurs in both structures, apparently due to close contacts between the heme and leucines 123 and 133, but increases on binding NO even though the steric contacts have not changed. We also report the structure of NP4 in complexes with histamine (1.50 A) and imidazole (1.27 A). Unexpectedly, two mobile loops that rearrange to pack against the bound NO in NP4-NO, also rearrange in the NP4-imidazole complex. This conformational change is apparently driven by the nonpolar nature of the NO and imidazole (as bound) ligands. Taken together, the desolvation of the NO binding pocket through a change in protein conformation, and the bending of the NO moiety, possibly through protein-assisted heme ruffling, may lead to a nitrosyl-heme complex that is unusually resistant to autoreduction.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, University of Arizona, Tucson, Arizona 85721, USA.