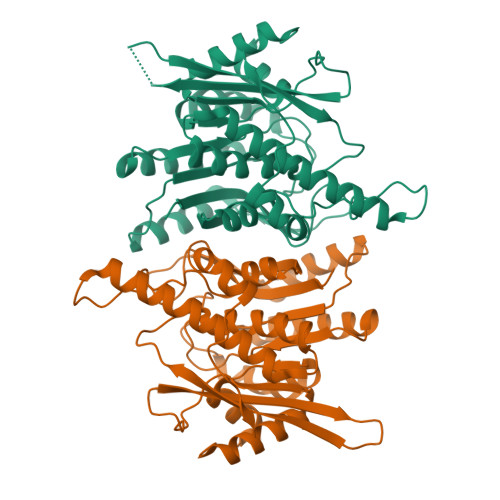
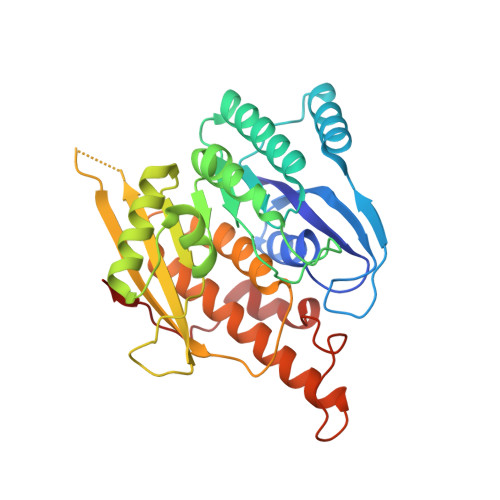
Crystal structure of brain pyridoxal kinase, a novel member of the ribokinase superfamily
Li, M.H., Kwok, F., Chang, W.R., Lau, C.K., Zhang, J.P., Lo, S.C., Jiang, T., Liang, D.C.(2002) J Biological Chem 277: 46385-46390
- PubMed: 12235162
- DOI: https://doi.org/10.1074/jbc.M208600200
- Primary Citation of Related Structures:
1LHP, 1LHR - PubMed Abstract:
The three-dimensional structures of brain pyridoxal kinase and its complex with the nucleotide ATP have been elucidated in the dimeric form at 2.1 and 2.6 A, respectively. Results have shown that pyridoxal kinase, as an enzyme obeying random sequential kinetics in catalysis, does not possess a lid shape structure common to all kinases in the ribokinase superfamily. This finding has been shown to be in line with the condition that pyridoxal kinase binds substrates with variable sizes of chemical groups at position 4 of vitamin B(6) and its derivatives. In addition, the enzyme contains a 12-residue peptide loop in the active site for the prevention of premature hydrolysis of ATP. Conserved amino acid residues Asp(118) and Tyr(127) in the peptide loop could be moved to a position covering the nucleotide after its binding so that its chance to hydrolyze in the aqueous environment of the active site was reduced. With respect to the evolutionary trend of kinase enzymes, the existence of this loop in pyridoxal kinase could be classified as an independent category in the ribokinase superfamily according to the structural feature found and mechanism followed in catalysis.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Science, Beijing 100101, China.