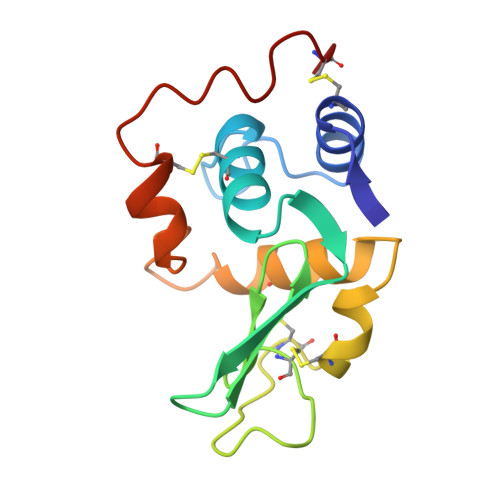
Refinement of human lysozyme at 1.5 A resolution analysis of non-bonded and hydrogen-bond interactions.
Artymiuk, P.J., Blake, C.C.(1981) J Mol Biology 152: 737-762
- PubMed: 7334520
- DOI: https://doi.org/10.1016/0022-2836(81)90125-x
- Primary Citation of Related Structures:
1LZ1