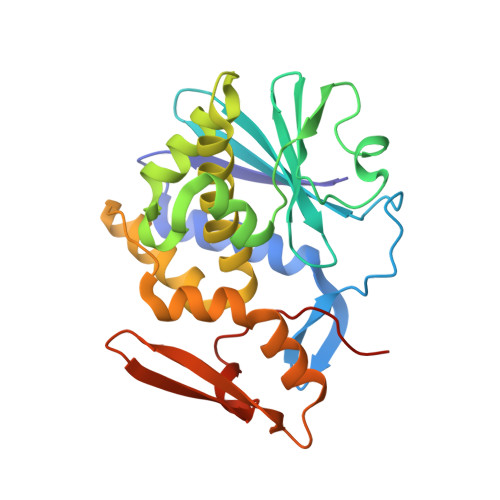
Crystallisation under microgravity of mistletoe lectin I from Viscum album with adenine monophosphate and the crystal structure at 1.9 A resolution.
Krauspenhaar, R., Rypniewski, W., Kalkura, N., Moore, K., DeLucas, L., Stoeva, S., Mikhailov, A., Voelter, W., Betzel, C.h.(2002) Acta Crystallogr D Biol Crystallogr 58: 1704-1707
- PubMed: 12351890
- DOI: https://doi.org/10.1107/s0907444902014270
- Primary Citation of Related Structures:
1M2T - PubMed Abstract:
The crystal structure of the ribosome-inactivating protein (RIP) mistletoe lectin I (ML-I) from Viscum album in complex with adenine has been refined to 1.9 A resolution. High quality crystals of the ML-I complex were obtained by the method of vapour diffusion using the high density protein crystal growth system (HDPCG) on the international space station, mission ISS 6A. Hexagonal crystals were grown during three months under microgravity conditions. Diffraction data to 1.9A were collected applying synchrotron radiation and cryo- techniques. The structure was refined subsequently to analyse the structure of ML-I and particularly the active site conformation, complexed by adenine that mimics the RNA substrate binding.
Organizational Affiliation:
Institute of Medical Biochemistry and Molecular Biology, University Hospital, c/o DESY, Build 22a, Notkestr 85, 22603 Hamburg, Germany.