Three-dimensional structure of a thermostable native cellobiohydrolase, CBH IB, and molecular characterization of the cel7 gene from the filamentous fungus, Talaromyces emersonii
Grassick, A., Murray, P.G., Thompson, R., Collins, C.M., Byrnes, L., Birrane, G., Higgins, T.M., Tuohy, M.G.(2004) Eur J Biochem 271: 4495-4506
- PubMed: 15560790
- DOI: https://doi.org/10.1111/j.1432-1033.2004.04409.x
- Primary Citation of Related Structures:
1Q9H - PubMed Abstract:
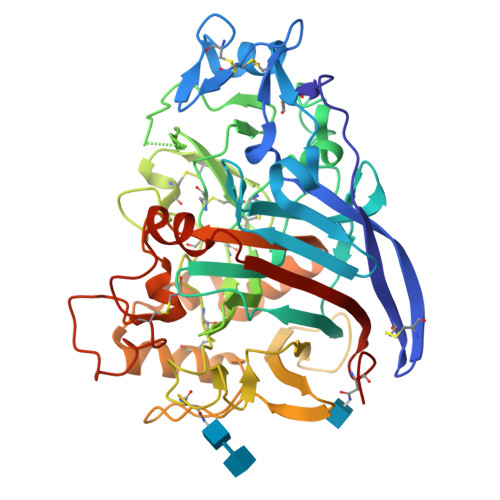
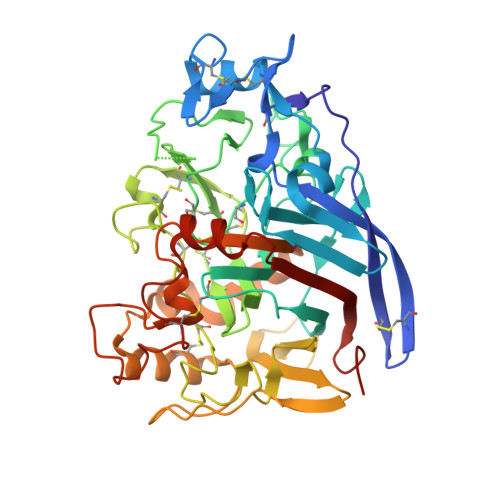
The X-ray structure of native cellobiohydrolase IB (CBH IB) from the filamentous fungus Talaromyces emersonii, PDB 1Q9H, was solved to 2.4 A by molecular replacement. 1Q9H is a glycoprotein that consists of a large, single domain with dimensions of approximately 60 A x 40 A x 50 A and an overall beta-sandwich structure, the characteristic fold of Family 7 glycosyl hydrolases (GH7). It is the first structure of a native glycoprotein and cellulase from this thermophilic eukaryote. The long cellulose-binding tunnel seen in GH7 Cel7A from Trichoderma reesei is conserved in 1Q9H, as are the catalytic residues. As a result of deletions and other changes in loop regions, the binding and catalytic properties of T. emersonii 1Q9H are different. The gene (cel7) encoding CBH IB was isolated from T. emersonii and expressed heterologously with an N-terminal polyHis-tag, in Escherichia coli. The deduced amino acid sequence of cel7 is homologous to fungal cellobiohydrolases in GH7. The recombinant cellobiohydrolase was virtually inactive against methylumberiferyl-cellobioside and chloronitrophenyl-lactoside, but partial activity could be restored after refolding of the urea-denatured enzyme. Profiles of cel7 expression in T. emersonii, investigated by Northern blot analysis, revealed that expression is regulated at the transcriptional level. Putative regulatory element consensus sequences for cellulase transcription factors have been identified in the upstream region of the cel7 genomic sequence.
Organizational Affiliation:
Molecular Glycobiotechnology Group, Department of Biochemistry, National University of Ireland, Galway, Ireland.