Human Glucose-6-Phosphate Dehydrogenase: The Crystal Structure Reveals a Structural Nadp+ Molecule and Provides Insights Into Enzyme Deficiency
Au, S.W.N., Gover, S., Lam, V.M.S., Adams, M.J.(2000) Structure 8: 293
- PubMed: 10745013
- DOI: https://doi.org/10.1016/s0969-2126(00)00104-0
- Primary Citation of Related Structures:
1QKI - PubMed Abstract:
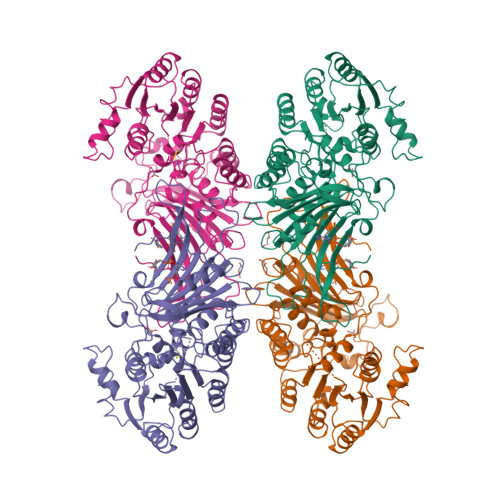
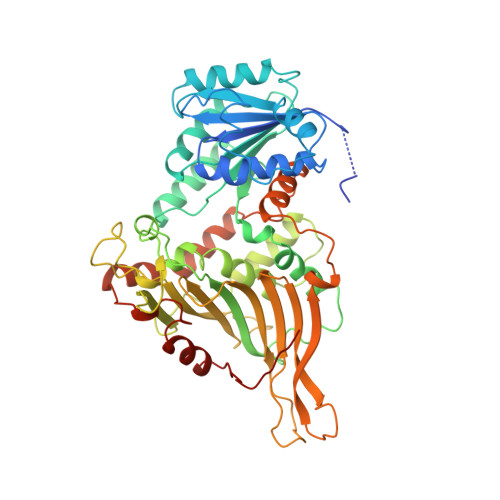
Glucose-6-phosphate dehydrogenase (G6PD) catalyses the first committed step in the pentose phosphate pathway; the generation of NADPH by this enzyme is essential for protection against oxidative stress. The human enzyme is in a dimer<-->tetramer equilibrium and its stability is dependent on NADP(+) concentration. G6PD deficiency results from many different point mutations in the X-linked gene encoding G6PD and is the most common human enzymopathy. Severe deficiency causes chronic non-spherocytic haemolytic anaemia; the usual symptoms are neonatal jaundice, favism and haemolytic anaemia. We have determined the first crystal structure of a human G6PD (the mutant Canton, Arg459-->Leu) at 3 A resolution. The tetramer is a dimer of dimers. Despite very similar dimer topology, there are two major differences from G6PD of Leuconostoc mesenteroides: a structural NADP(+) molecule, close to the dimer interface but integral to the subunit, is visible in all subunits of the human enzyme; and an intrasubunit disulphide bond tethers the otherwise disordered N-terminal segment. The few dimer-dimer contacts making the tetramer are charge-charge interactions. The importance of NADP(+) for stability is explained by the structural NADP(+) site, which is not conserved in prokaryotes. The structure shows that point mutations causing severe deficiency predominate close to the structural NADP(+) and the dimer interface, primarily affecting the stability of the molecule. They also indicate that a stable dimer is essential to retain activity in vivo. As there is an absolute requirement for some G6PD activity, residues essential for coenzyme or substrate binding are rarely modified.
Organizational Affiliation:
Laboratory of Molecular Biophysics, Department of Biochemistry, University of Oxford, The University of Hong Kong, Department of Biochemistry, Oxford, OX1 3QU, UK, Hong Kong.