Phylogenetic classification of protozoa based on the structure of the linker domain in the bifunctional enzyme, dihydrofolate reductase-thymidylate synthase
O'Neil, R.H., Lilien, R.H., Donald, B.R., Stroud, R.M., Anderson, A.C.(2003) J Biological Chem 278: 52980-52987
- PubMed: 14555647
- DOI: https://doi.org/10.1074/jbc.M310328200
- Primary Citation of Related Structures:
1QZF - PubMed Abstract:
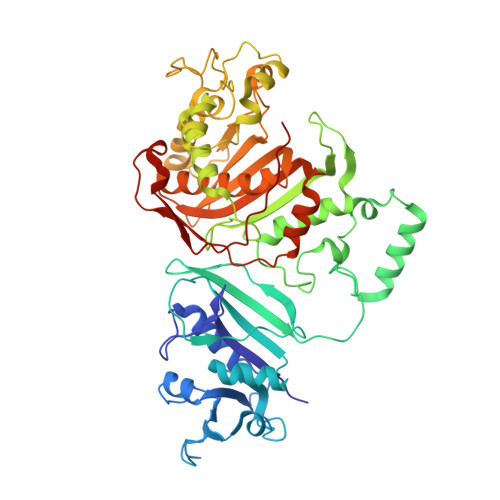
We have determined the crystal structure of dihydrofolate reductase-thymidylate synthase (DHFR-TS) from Cryptosporidium hominis, revealing a unique linker domain containing an 11-residue alpha-helix that has extensive interactions with the opposite DHFR-TS monomer of the homodimeric enzyme. Analysis of the structure of DHFR-TS from C. hominis and of previously solved structures of DHFR-TS from Plasmodium falciparum and Leishmania major reveals that the linker domain primarily controls the relative orientation of the DHFR and TS domains. Using the tertiary structure of the linker domains, we have been able to place a number of protozoa in two distinct and dissimilar structural families corresponding to two evolutionary families and provide the first structural evidence validating the use of DHFR-TS as a tool of phylogenetic classification. Furthermore, the structure of C. hominis DHFR-TS calls into question surface electrostatic channeling as the universal means of dihydrofolate transport between TS and DHFR in the bifunctional enzyme.
Organizational Affiliation:
Department of Chemistry, Dartmouth College, Hanover, New Hampshire 03755, USA.