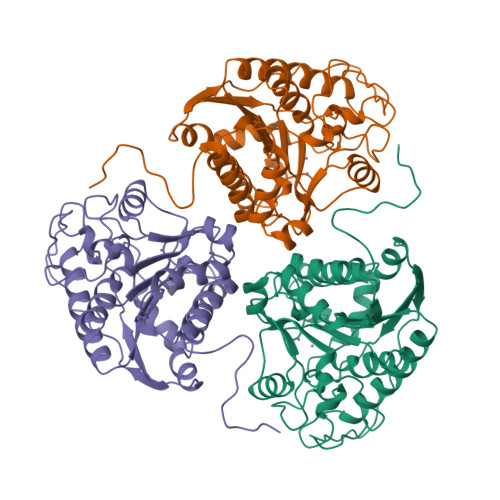
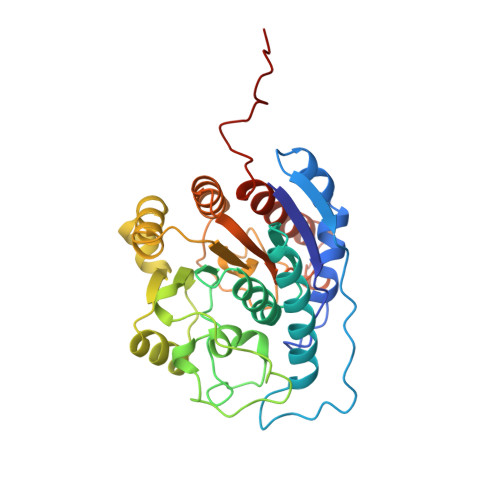
Structure of a unique binuclear manganese cluster in arginase.
Kanyo, Z.F., Scolnick, L.R., Ash, D.E., Christianson, D.W.(1996) Nature 383: 554-557
- PubMed: 8849731
- DOI: https://doi.org/10.1038/383554a0
- Primary Citation of Related Structures:
1RLA - PubMed Abstract:
Each individual excretes roughly 10 kg of urea per year, as a result of the hydrolysis of arginine in the final cytosolic step of the urea cycle. This reaction allows the disposal of nitrogenous waste from protein catabolism, and is catalysed by the liver arginase enzyme. In other tissues that lack a complete urea cycle, arginase regulates cellular arginine and ornithine concentrations for biosynthetic reactions, including nitric oxide synthesis: in the macrophage, arginase activity is reciprocally coordinated with that of NO synthase to modulate NO-dependent cytotoxicity. The bioinorganic chemistry of arginase is particularly rich because this enzyme is one of very few that specifically requires a spin-coupled Mn2+-Mn2+ cluster for catalytic activity in vitro and in vivo. The 2.1 angstrom-resolution crystal structure of trimeric rat liver arginase reveals that this unique metal cluster resides at the bottom of an active-site cleft that is 15 angstroms deep. Analysis of the structure indicates that arginine hydrolysis is achieved by a metal-activated solvent molecule which symmetrically bridges the two Mn2+ ions.
Organizational Affiliation:
Department of Chemistry, University of Pennsylvania, Philadelphia 19104-6323, USA.