Crystal structures of the liganded and unliganded nickel-binding protein NikA from Escherichia coli
Heddle, J., Scott, D.J., Unzai, S., Park, S.-Y., Tame, J.R.H.(2003) J Biological Chem 278: 50322-50329
- PubMed: 12960164
- DOI: https://doi.org/10.1074/jbc.M307941200
- Primary Citation of Related Structures:
1UIU, 1UIV - PubMed Abstract:
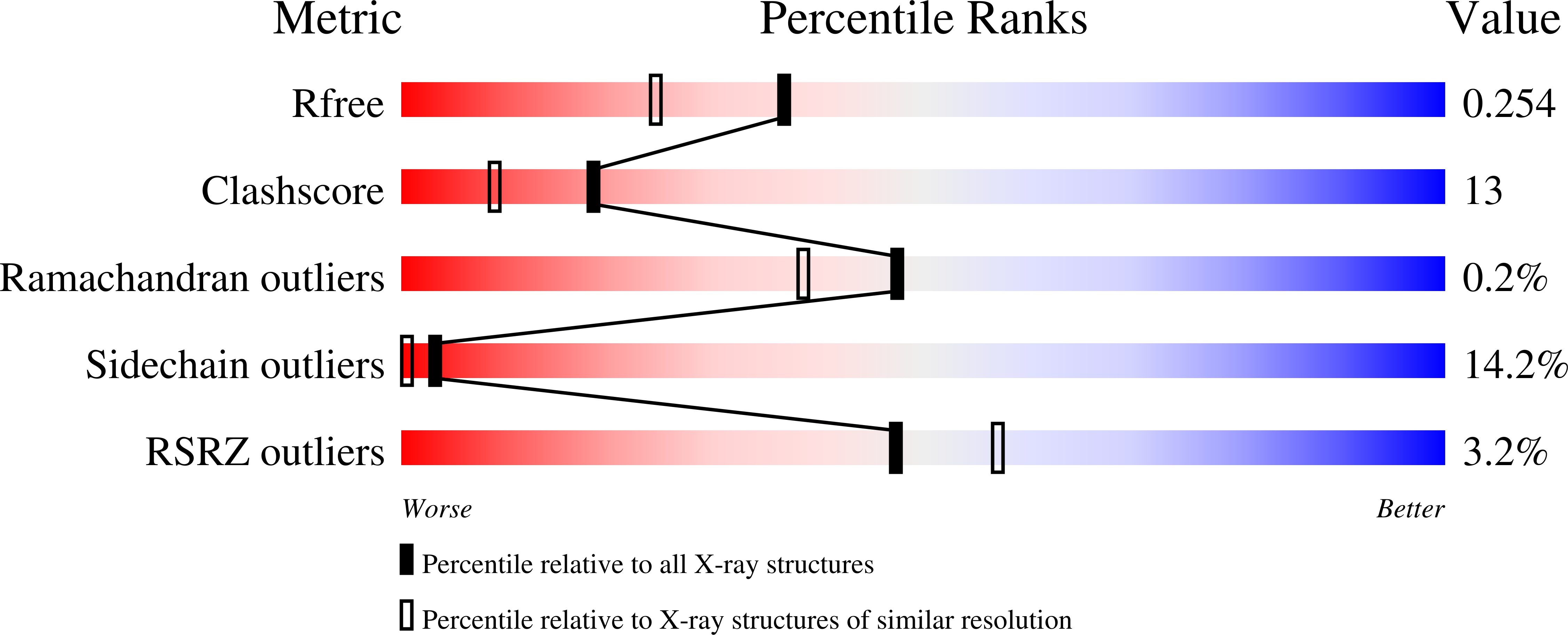
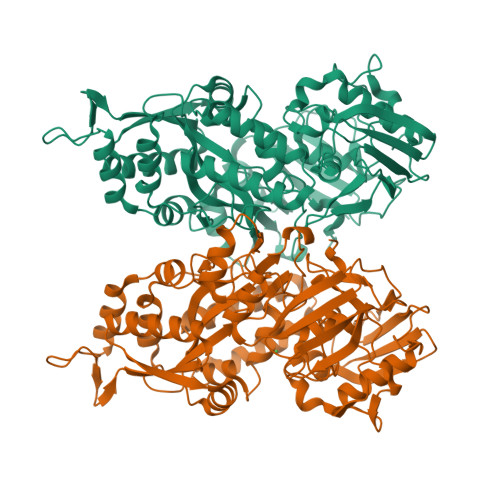
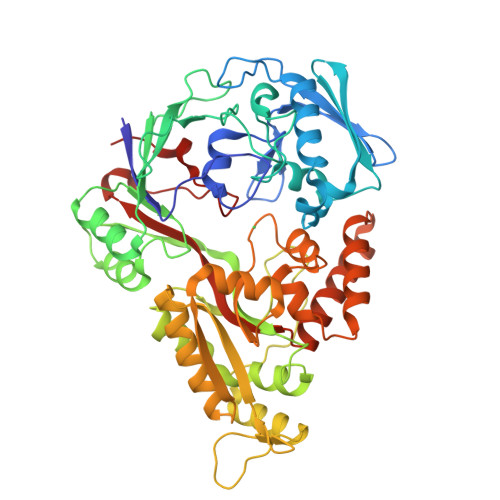
Bacteria have evolved a number of tightly controlled import and export systems to maintain intracellular levels of the essential but potentially toxic metal nickel. Nickel homeostasis systems include the dedicated nickel uptake system nik found in Escherichia coli, a member of the ABC family of transporters, that involves a periplasmic nickel-binding protein, NikA. This is the initial nickel receptor and mediator of the chemotactic response away from nickel. We have solved the crystal structure of NikA protein in the presence and absence of nickel, showing that it behaves as a "classical" periplasmic binding protein. In contrast to other binding proteins, however, the ligand remains accessible to the solvent and is not completely enclosed. No direct bonds are formed between the metal cation and the protein. The nickel binding site is apolar, quite unlike any previously characterized protein nickel binding site. Despite relatively weak binding, NikA is specific for nickel. Using isothermal titration calorimetry, the dissociation constant for nickel was found to be approximately 10 microm and that for cobalt was approximately 20 times higher.
Organizational Affiliation:
Protein Design Laboratory, Yokohama City University, Tsurumi, Suehiro 1-7-29, Yokohama 230-0045, Japan.