Crystal structures of Fe2+ dioxygenase superoxo, alkylperoxo, and bound product intermediates
Kovaleva, E.G., Lipscomb, J.D.(2007) Science 316: 453-457
- PubMed: 17446402
- DOI: https://doi.org/10.1126/science.1134697
- Primary Citation of Related Structures:
2IG9, 2IGA - PubMed Abstract:
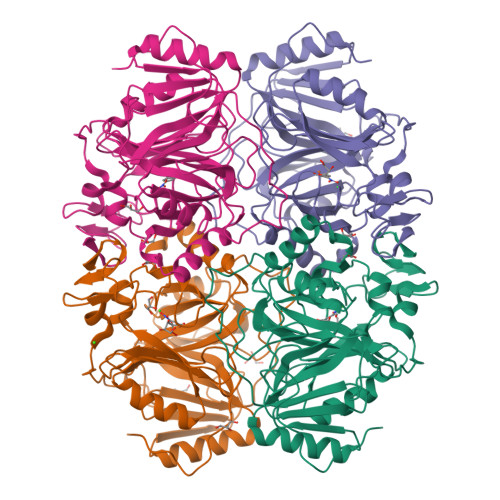
We report the structures of three intermediates in the O2 activation and insertion reactions of an extradiol ring-cleaving dioxygenase. A crystal of Fe2+-containing homoprotocatechuate 2,3-dioxygenase was soaked in the slow substrate 4-nitrocatechol in a low O2 atmosphere. The x-ray crystal structure shows that three different intermediates reside in different subunits of a single homotetrameric enzyme molecule. One of these is the key substrate-alkylperoxo-Fe2+ intermediate, which has been predicted, but not structurally characterized, in an oxygenase. The intermediates define the major chemical steps of the dioxygenase mechanism and point to a general mechanistic strategy for the diverse 2-His-1-carboxylate enzyme family.
Organizational Affiliation:
Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, MN 55455, USA.