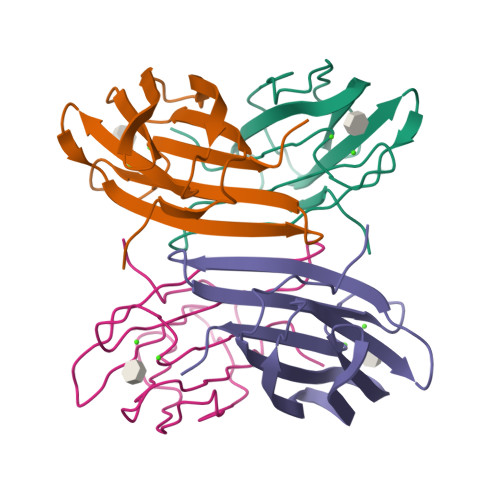
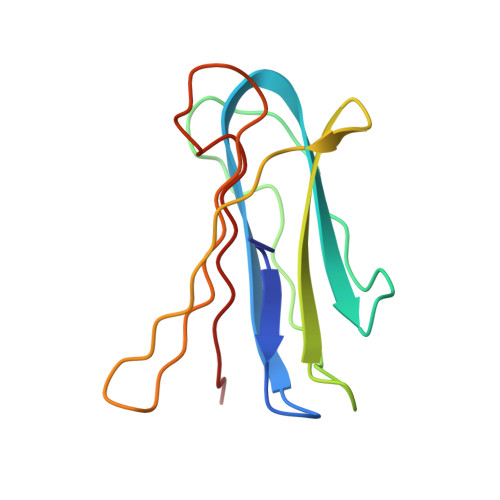
Engineering of Pa-Iil Lectin from Pseudomonas Aeruginosa - Unravelling the Role of the Specificity Loop for Sugar Preference.
Adam, J., Pokorna, M., Sabin, C., Mitchell, E.P., Imberty, A., Wimmerova, M.(2007) BMC Struct Biol 7: 36
- PubMed: 17540045
- DOI: https://doi.org/10.1186/1472-6807-7-36
- Primary Citation of Related Structures:
2JDM, 2JDN, 2JDP, 2JDU, 2JDY - PubMed Abstract:
Lectins are proteins of non-immune origin capable of binding saccharide structures with high specificity and affinity. Considering the high encoding capacity of oligosaccharides, this makes lectins important for adhesion and recognition. The present study is devoted to the PA-IIL lectin from Pseudomonas aeruginosa, an opportunistic human pathogen capable of causing lethal complications in cystic fibrosis patients. The lectin may play an important role in the process of virulence, recognizing specific saccharide structures and subsequently allowing the bacteria to adhere to the host cells. It displays high values of affinity towards monosaccharides, especially fucose--a feature caused by unusual binding mode, where two calcium ions participate in the interaction with saccharide. Investigating and understanding the nature of lectin-saccharide interactions holds a great potential of use in the field of drug design, namely the targeting and delivery of active compounds to the proper site of action. In vitro site-directed mutagenesis of the PA-IIL lectin yielded three single point mutants that were investigated both structurally (by X-ray crystallography) and functionally (by isothermal titration calorimetry). The mutated amino acids (22-23-24 triad) belong to the so-called specificity binding loop responsible for the monosaccharide specificity of the lectin. The mutation of the amino acids resulted in changes to the thermodynamic behaviour of the mutants and subsequently in their relative preference towards monosaccharides. Correlation of the measured data with X-ray structures provided the molecular basis for rationalizing the affinity changes. The mutations either prevent certain interactions to be formed or allow formation of new interactions--both of afore mentioned have strong effects on the saccharide preferences. Mutagenesis of amino acids forming the specificity binding loop allowed identification of one amino acid that is crucial for definition of the lectin sugar preference. Altering specificity loop amino acids causes changes in saccharide-binding preferences of lectins derived from PA-IIL, via creation or blocking possible binding interactions. This finding opens a gate towards protein engineering and subsequent protein design to refine the desired binding properties and preferences, an approach that could have strong potential for drug design.
Organizational Affiliation:
National Centre for Biomolecular Research, Faculty of Science, Masaryk University, Kotlarska 2, Brno, Czech Republic. honzadam@chemi.muni.cz