A high-resolution structure of ligand-free human glutamate carboxypeptidase II.
Barinka, C., Starkova, J., Konvalinka, J., Lubkowski, J.(2007) Acta Crystallogr Sect F Struct Biol Cryst Commun 63: 150-153
- PubMed: 17329803
- DOI: https://doi.org/10.1107/S174430910700379X
- Primary Citation of Related Structures:
2OOT - PubMed Abstract:
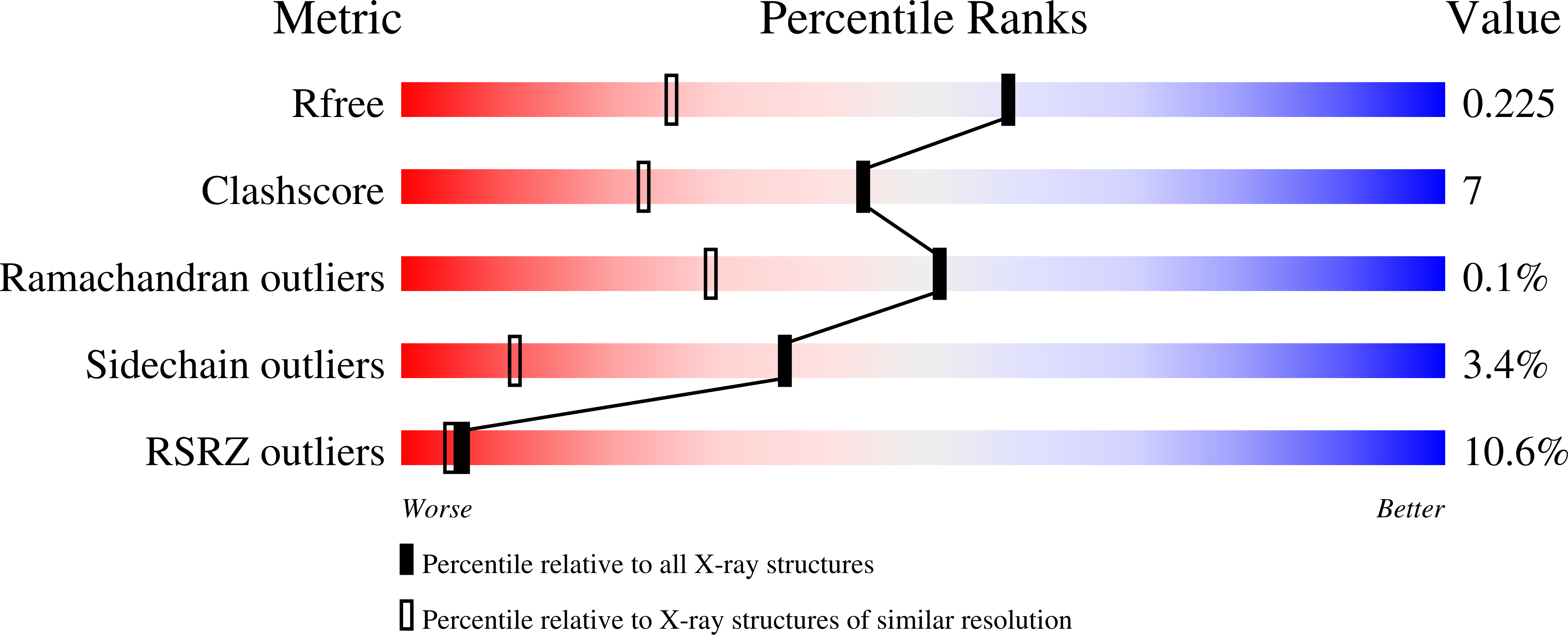
Human glutamate carboxypeptidase II (GCPII; EC 3.4.17.21) is an established marker for prostate-cancer diagnosis as well as a candidate therapeutic target for the treatment of diverse pathologies that involve glutamatergic transmission. Structural data on GCPII are thus valuable for the design and optimization of GCPII-specific inhibitors and diagnostic probes. The currently available structure of ligand-free GCPII was refined to a resolution of 3.5 A. This work reports the structure of the protein refined to 1.65 A resolution, with crystallographic values of R = 0.207 and R(free) = 0.228. The new structure extends the resolution appreciably and the new model based on this data shows significant differences when compared with the previously published model.
Organizational Affiliation:
National Cancer Institute at Frederick, Center for Cancer Research, Frederick, MD 21702, USA. cyril@ncifcrf.gov