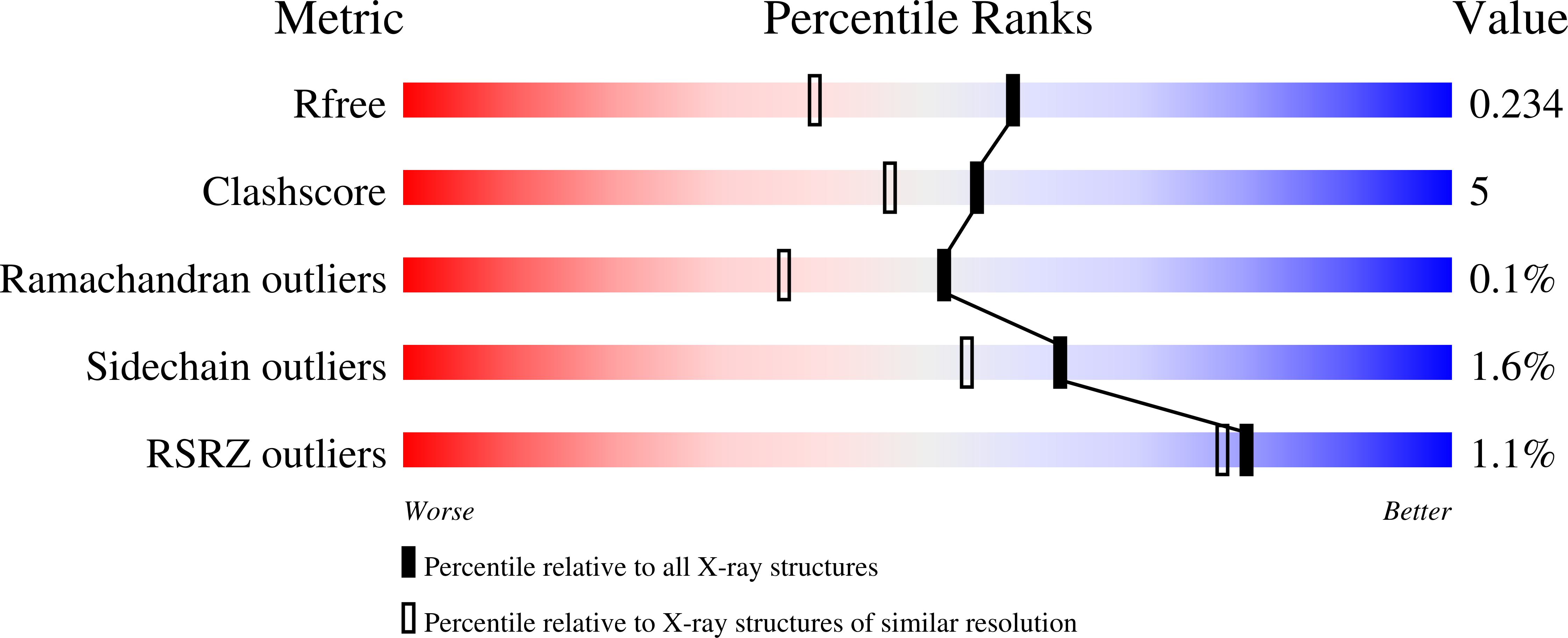
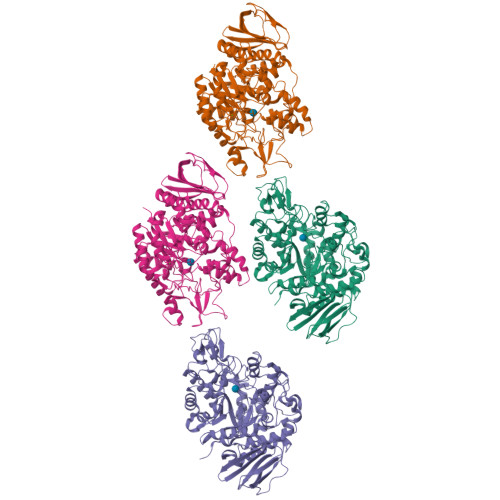
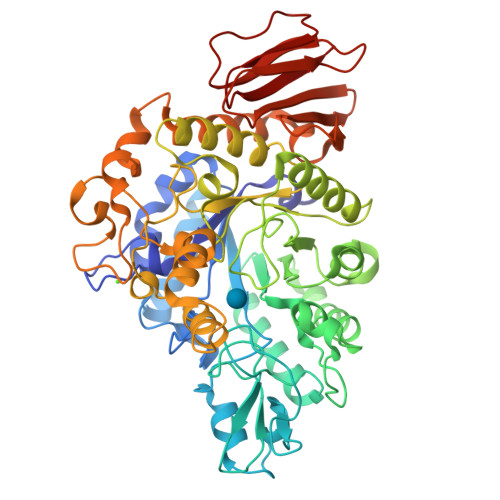
Trehalulose synthase native and carbohydrate complexed structures provide insights into sucrose isomerization.
Ravaud, S., Robert, X., Watzlawick, H., Haser, R., Mattes, R., Aghajari, N.(2007) J Biological Chem 61: 100-103
- PubMed: 17597061
- DOI: https://doi.org/10.1074/jbc.M704515200
- Primary Citation of Related Structures:
1ZJA, 2PWD, 2PWE, 2PWF, 2PWG, 2PWH - PubMed Abstract:
Various diseases related to the overconsumption of sugar make a growing need for sugar substitutes. Because sucrose is an inexpensive and readily available d-glucose donor, the industrial potential for enzymatic synthesis of the sucrose isomers trehalulose and/or isomaltulose from sucrose is large. The product specificity of sucrose isomerases that catalyze this reaction depends essentially on the possibility for tautomerization of sucrose, which is required for trehalulose formation. For optimal use of the enzyme, targeting controlled synthesis of these functional isomers, it is necessary to minimize the side reactions. This requires an extensive analysis of substrate binding modes and of the specificity-determining sites in the structure. The 1.6-2.2-A resolution three-dimensional structures of native and mutant complexes of a trehalulose synthase from Pseudomonas mesoacidophila MX-45 mimic successive states of the enzyme reaction. Combined with mutagenesis studies they give for the first time thorough insights into substrate recognition and processing and reaction specificities of these enzymes. Among the important outcomes of this study is the revelation of an aromatic clamp defined by Phe(256) and Phe(280) playing an essential role in substrate recognition and in controlling the reaction specificity, which is further supported by mutagenesis studies. Furthermore, this study highlights essential residues for binding the glucosyl and fructosyl moieties. The introduction of subtle changes informed by comparative three-dimensional structural data observed within our study can lead to fundamental modifications in the mode of action of sucrose isomerases and hence provide a template for industrial catalysts.
Organizational Affiliation:
Laboratoire de BioCristallographie, Institut de Biologie et Chimie des Protéines, CNRS et Université de Lyon, UMR 5086, IFR 128 BioSciences Gerland-Lyon Sud, F-69367 Lyon Cedex 07, France.