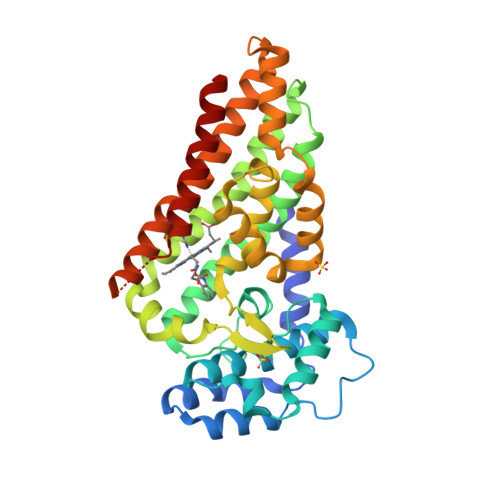
The Second Enzyme in Pyrrolnitrin Biosynthetic Pathway is Related to the Heme-Dependent Dioxygenase Superfamily
De Laurentis, W., Khim, L., Anderson, J.L.R., Adam, A., Phillips, R.S., Chapman, S.K., Van Pee, K.-H., Naismith, J.H.(2007) Biochemistry 46: 12393
- PubMed: 17924666
- DOI: https://doi.org/10.1021/bi7012189
- Primary Citation of Related Structures:
2V7I, 2V7J, 2V7K, 2V7L, 2V7M - PubMed Abstract:
Pyrrolnitrin is a commonly used and clinically effective treatment for fungal infections and provides the structural basis for the more widely used fludioxinil. The pyrrolnitrin biosynthetic pathway consists of four chemical steps, the second of which is the rearrangement of 7-chloro-tryptophan by the enzyme PrnB, a reaction that is so far unprecedented in biochemistry. When expressed in Pseudomonas fluorescens, PrnB is red in color due to the fact that it contains 1 mol of heme b per mole of protein. The crystal structure unexpectedly establishes PrnB as a member of the heme-dependent dioxygenase superfamily with significant structural but not sequence homology to the two-domain indoleamine 2,3-dioxygenase enzyme (IDO). The heme-binding domain is also structurally similar to that of tryptophan 2,3-dioxygenase (TDO). Here we report the binary complex structures of PrnB with d- and l-tryptophan and d- and l-7-chloro-tryptophan. The structures identify a common hydrophobic pocket for the indole ring but exhibit unusual heme ligation and substrate binding when compared with that observed in the TDO crystal structures. Our solution studies support the heme ligation observed in the crystal structures. Purification of the hexahistidine-tagged PrnB yields homogeneous protein that only displays in vitro activity with 7-chloro-l-tryptophan after reactivation with crude extract from the host strain, suggesting that an as yet unknown cofactor is required for activity. Mutation of the proximal heme ligand results, not surprisingly, in inactive enzyme. Redox titrations show that PrnB displays a significantly different reduction potential to that of IDO or TDO, indicating possible differences in the PrnB catalytic cycle. This is confirmed by the absence of tryptophan dioxygenase activity in PrnB, although a stable oxyferrous adduct (which is the first intermediate in the TDO/IDO catalytic cycle) can be generated. We propose that PrnB shares a key catalytic step with TDO and IDO, generation of a tryptophan hydroperoxide intermediate, although this species suffers a different fate in PrnB, leading to the eventual formation of the product, monodechloroaminopyrrolnitrin.
Organizational Affiliation:
Centre for Biomolecular Sciences, EastChem, The University, St Andrews, Scotland, KY16 9ST, UK.