Crystal Structure of Bacillus Subtilis Spp1 Phage Gp22 Shares Fold Similarity with a Domain of Lactococcal Phage P2 Rbp.
Veesler, D., Blangy, S., Spinelli, S., Tavares, P., Campanacci, V., Cambillau, C.(2010) Protein Sci 19: 1439
- PubMed: 20506290
- DOI: https://doi.org/10.1002/pro.416
- Primary Citation of Related Structures:
2XC8 - PubMed Abstract:
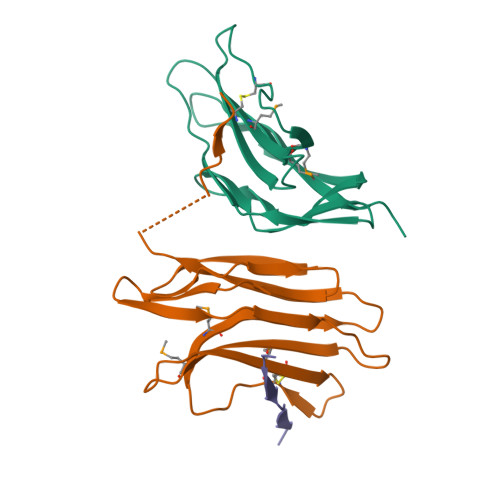
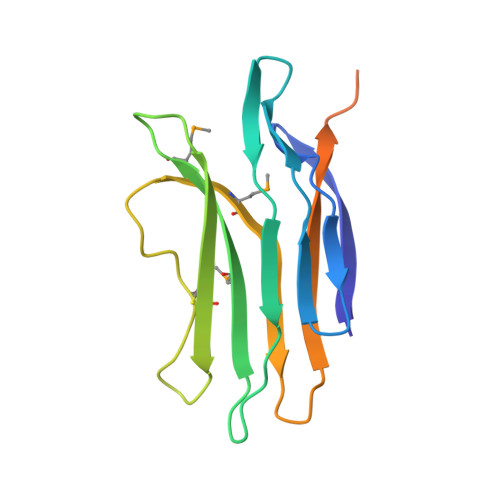
SPP1 is a siphophage infecting the gram-positive bacterium Bacillus subtilis. It is constituted by an icosahedric head and a long non-contractile tail formed by gene products (gp) 17-21. A group of 5 small genes (gp 22-24.1) follows in the genome those coding for the main tail components. However, the belonging of the corresponding gp to the tail or to other parts of the phage is not documented. Among these, gp22 lacks sequence identity to any known protein. We report here the gp22 structure solved by X-ray crystallography at 2.35 A resolution. We found that gp22 is a monomer in solution and possesses a significant structural similarity with lactococcal phage p2 ORF 18 N-terminal "shoulder" domain.
Organizational Affiliation:
Architecture et Fonction des Macromolécules Biologiques, UMR 6098 CNRS and Universités d'Aix-Marseille I and II, Campus de Luminy, Case 932, 13288 Marseille Cedex 09, France.