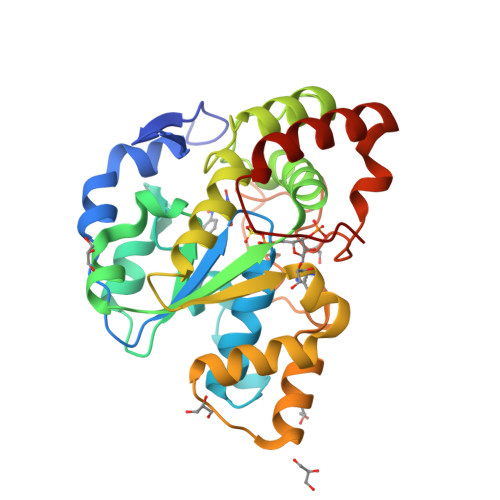
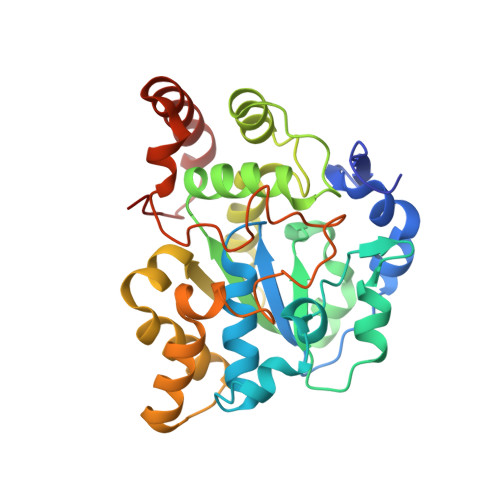
Snapshot of a Michaelis complex in a sulfuryl transfer reaction: Crystal structure of a mouse sulfotransferase, mSULT1D1, complexed with donor substrate and accepter substrate
Teramoto, T., Sakakibara, Y., Liu, M.-C., Suiko, M., Kimura, M., Kakuta, Y.(2009) Biochem Biophys Res Commun 383: 83-87
- PubMed: 19344693
- DOI: https://doi.org/10.1016/j.bbrc.2009.03.146
- Primary Citation of Related Structures:
2ZYT, 2ZYU, 2ZYV, 2ZYW - PubMed Abstract:
We report the crystal structure of mouse sulfotransferase, mSULT1D1, complexed with donor substrate 3'-phosphoadenosine 5'-phosphosulfate and accepter substrate p-nitrophenol. The structure is the first report of the native Michaelis complex of sulfotransferase. In the structure, three proposed catalytic residues (Lys48, Lys106, and His108) were in proper positions for engaging in the sulfuryl transfer reaction. The data strongly support that the sulfuryl transfer reaction proceeds through an S(N)2-like in-line displacement mechanism.
Organizational Affiliation:
Department of Systems Life Sciences, Kyushu University, Hakozaki, Higashi-ku, Fukuoka, Japan.