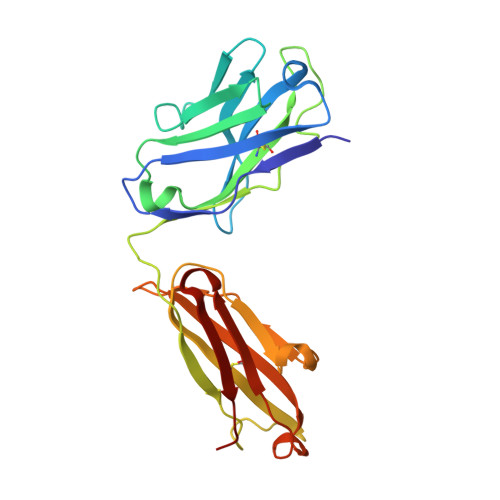
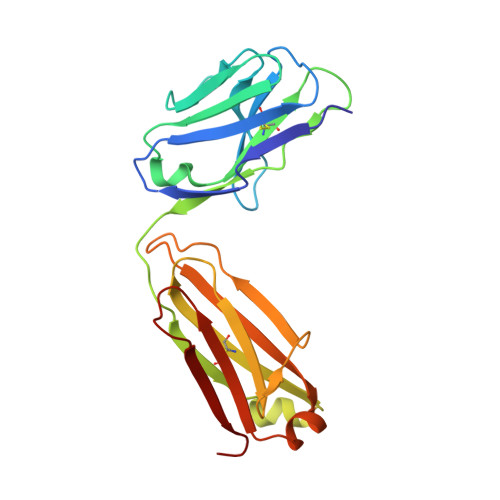
The crystal structure of sphingosine-1-phosphate in complex with a Fab fragment reveals metal bridging of an antibody and its antigen.
Wojciak, J.M., Zhu, N., Schuerenberg, K.T., Moreno, K., Shestowsky, W.S., Hiraiwa, M., Sabbadini, R., Huxford, T.(2009) Proc Natl Acad Sci U S A 106: 17717-17722
- PubMed: 19815502
- DOI: https://doi.org/10.1073/pnas.0906153106
- Primary Citation of Related Structures:
3I9G - PubMed Abstract:
The pleiotropic signaling lipid sphingosine-1-phosphate (S1P) plays significant roles in angiogenesis, heart disease, and cancer. LT1009 (also known as sonepcizumab) is a humanized monoclonal antibody that binds S1P with high affinity and specificity. Because the antibody is currently in clinical trials, it is important to confirm by structural and biochemical analyses that it binds its target in a predictable manner. Therefore, we determined the structure of a complex between the LT1009 antibody Fab fragment and S1P refined to 1.90 A resolution. The antibody employs unique and diverse strategies to recognize its antigen. Two metal ions bridge complementarity determining regions from the antibody light chain and S1P. The coordination geometry, inductively coupled plasma spectroscopy, surface plasmon resonance spectroscopy, and biochemical assays suggest that these are Ca(2+). The amino alcohol head group of the sphingosine backbone is recognized through hydrogen bonding interactions from 1 aa side chain and polypeptide backbone atoms of the antibody light and heavy chains. The S1P hydrophobic tail is almost completely enclosed within a hydrophobic channel formed primarily by the heavy chain. Both treatment of the complex with metal chelators and mutation of amino acids in the light chain that coordinate the metal atoms or directly contact the polar head group abrogate binding, while mutations within the hydrophobic cavity also decrease S1P binding affinity. The structure suggests mechanistic details for recognition of a signaling lipid by a therapeutic antibody candidate. Moreover, this study provides direct structural evidence that antibodies are capable of using metals to bridge antigen:antibody complexes.
Organizational Affiliation:
Lpath Inc., 6335 Ferris Square, Suite A, San Diego, CA 92121, USA.