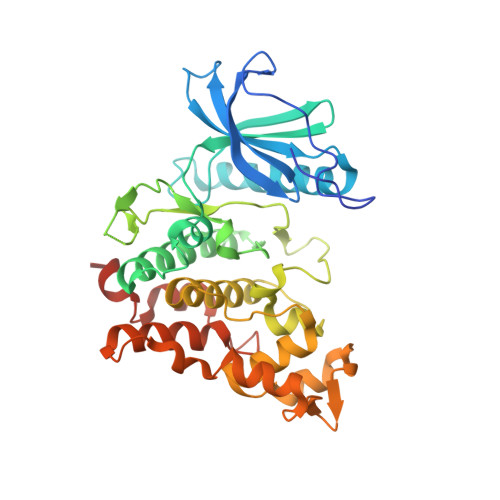
Structure of human CDC2-like kinase 2 (CLK2)
Chaikuad, A., Savitsky, P., Krojer, T., Muniz, J.R.C., Filippakopoulos, P., Rellos, P., Keates, T., Fedorov, O., Pike, A.C.W., Eswaran, J., Berridge, G., Phillips, C., Zhang, Y., von Delft, F., Weigelt, J., Arrowsmith, C.H., Edwards, A.M., Bountra, C., Knapp, S., Structural Genomics Consortium (SGC)To be published.