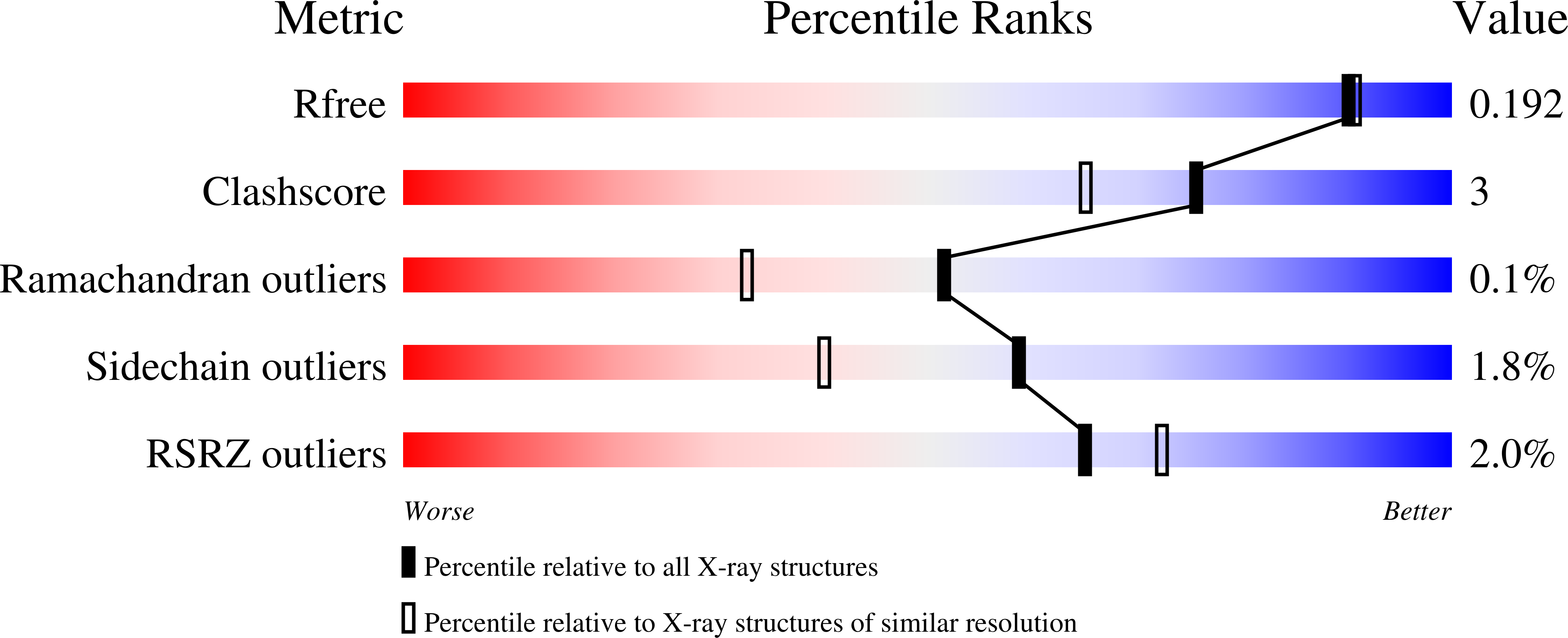
Bacillus cereus Phosphopentomutase Is an Alkaline Phosphatase Family Member That Exhibits an Altered Entry Point into the Catalytic Cycle.
Panosian, T.D., Nannemann, D.P., Watkins, G.R., Phelan, V.V., McDonald, W.H., Wadzinski, B.E., Bachmann, B.O., Iverson, T.M.(2011) J Biol Chem 286: 8043-8054
- PubMed: 21193409
- DOI: https://doi.org/10.1074/jbc.M110.201350
- Primary Citation of Related Structures:
3M8W, 3M8Y, 3M8Z, 3OT9 - PubMed Abstract:
Bacterial phosphopentomutases (PPMs) are alkaline phosphatase superfamily members that interconvert α-D-ribose 5-phosphate (ribose 5-phosphate) and α-D-ribose 1-phosphate (ribose 1-phosphate). We investigated the reaction mechanism of Bacillus cereus PPM using a combination of structural and biochemical studies. Four high resolution crystal structures of B. cereus PPM revealed the active site architecture, identified binding sites for the substrate ribose 5-phosphate and the activator α-D-glucose 1,6-bisphosphate (glucose 1,6-bisphosphate), and demonstrated that glucose 1,6-bisphosphate increased phosphorylation of the active site residue Thr-85. The phosphorylation of Thr-85 was confirmed by Western and mass spectroscopic analyses. Biochemical assays identified Mn(2+)-dependent enzyme turnover and demonstrated that glucose 1,6-bisphosphate treatment increases enzyme activity. These results suggest that protein phosphorylation activates the enzyme, which supports an intermolecular transferase mechanism. We confirmed intermolecular phosphoryl transfer using an isotope relay assay in which PPM reactions containing mixtures of ribose 5-[(18)O(3)]phosphate and [U-(13)C(5)]ribose 5-phosphate were analyzed by mass spectrometry. This intermolecular phosphoryl transfer is seemingly counter to what is anticipated from phosphomutases employing a general alkaline phosphatase reaction mechanism, which are reported to catalyze intramolecular phosphoryl transfer. However, the two mechanisms may be reconciled if substrate encounters the enzyme at a different point in the catalytic cycle.
Organizational Affiliation:
From the Departments of Pharmacology and.