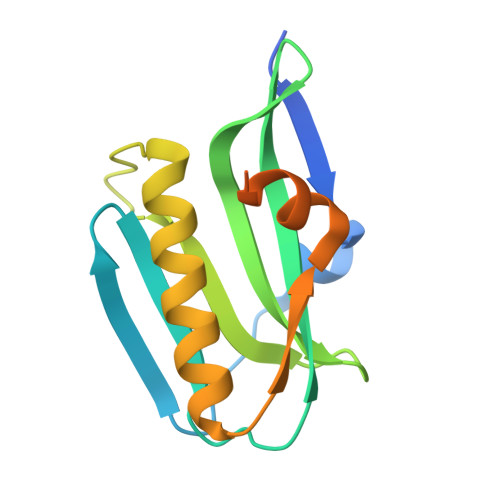
Conservation of a crystallographic interface suggests a role for beta-sheet augmentation in influenza virus NS1 multifunctionality.
Kerry, P.S., Long, E., Taylor, M.A., Russell, R.J.(2011) Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 858-861
- PubMed: 21821881
- DOI: https://doi.org/10.1107/S1744309111019312
- Primary Citation of Related Structures:
3RVC - PubMed Abstract:
The effector domain (ED) of the influenza virus virulence factor NS1 is capable of interaction with a variety of cellular and viral targets, although regulation of these events is poorly understood. Introduction of a W187A mutation into the ED abolishes dimer formation; however, strand-strand interactions between mutant NS1 ED monomers have been observed in two previous crystal forms. A new condition for crystallization of this protein [0.1 M Bis-Tris pH 6.0, 0.2 M NaCl, 22%(w/v) PEG 3350, 20 mM xylitol] was discovered using the hanging-drop vapour-diffusion method. Diffraction data extending to 1.8 Å resolution were collected from a crystal grown in the presence of 40 mM thieno[2,3-b]pyridin-2-ylmethanol. It was observed that there is conservation of the strand-strand interface in crystals of this monomeric NS1 ED in three different space groups. This observation, coupled with conformational changes in the interface region, suggests a potential role for β-sheet augmentation in NS1 function.
Organizational Affiliation:
Biomedical Sciences Research Complex, University of St Andrews, St Andrews, Fife, Scotland. psk5@st-andrews.ac.uk