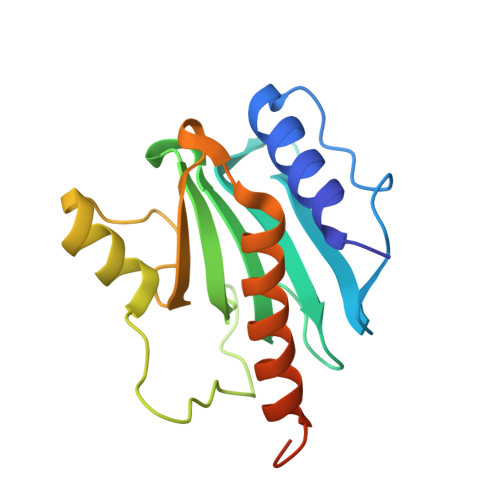
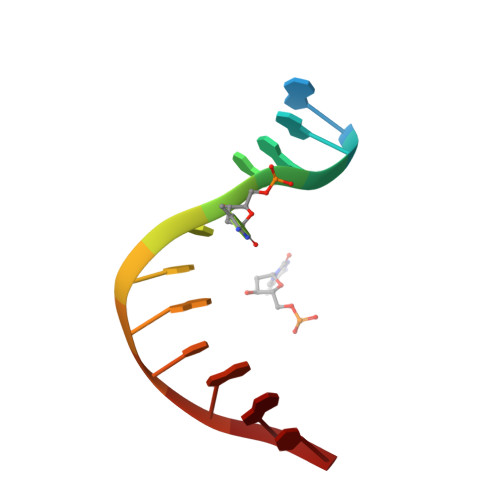
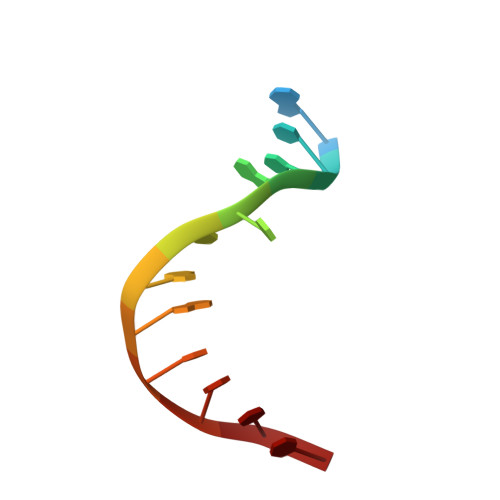
The recognition domain of the methyl-specific endonuclease McrBC flips out 5-methylcytosine.
Sukackaite, R., Grazulis, S., Tamulaitis, G., Siksnys, V.(2012) Nucleic Acids Res 40: 7552-7562
- PubMed: 22570415
- DOI: https://doi.org/10.1093/nar/gks332
- Primary Citation of Related Structures:
3SSC, 3SSD, 3SSE - PubMed Abstract:
DNA cytosine methylation is a widespread epigenetic mark. Biological effects of DNA methylation are mediated by the proteins that preferentially bind to 5-methylcytosine (5mC) in different sequence contexts. Until now two different structural mechanisms have been established for 5mC recognition in eukaryotes; however, it is still unknown how discrimination of the 5mC modification is achieved in prokaryotes. Here we report the crystal structure of the N-terminal DNA-binding domain (McrB-N) of the methyl-specific endonuclease McrBC from Escherichia coli. The McrB-N protein shows a novel DNA-binding fold adapted for 5mC-recognition. In the McrB-N structure in complex with methylated DNA, the 5mC base is flipped out from the DNA duplex and positioned within a binding pocket. Base flipping elegantly explains why McrBC system restricts only T4-even phages impaired in glycosylation [Luria, S.E. and Human, M.L. (1952) A nonhereditary, host-induced variation of bacterial viruses. J. Bacteriol., 64, 557-569]: flipped out 5-hydroxymethylcytosine is accommodated in the binding pocket but there is no room for the glycosylated base. The mechanism for 5mC recognition employed by McrB-N is highly reminiscent of that for eukaryotic SRA domains, despite the differences in their protein folds.
Organizational Affiliation:
Department of Protein-DNA Interactions, Institute of Biotechnology, Vilnius University, Graiciuno 8, 02241 Vilnius, Lithuania.