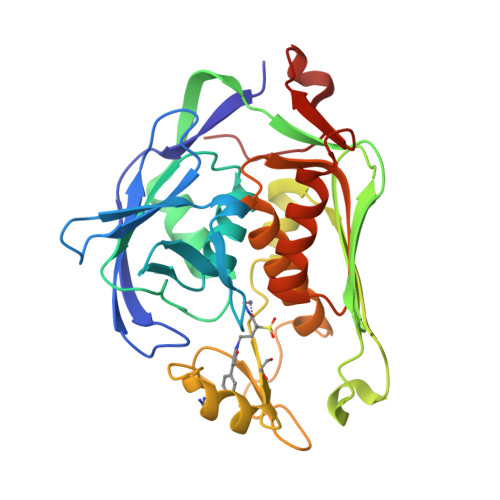
Pyridone methylsulfone hydroxamate LpxC inhibitors for the treatment of serious gram-negative infections.
Montgomery, J.I., Brown, M.F., Reilly, U., Price, L.M., Abramite, J.A., Arcari, J., Barham, R., Che, Y., Chen, J.M., Chung, S.W., Collantes, E.M., Desbonnet, C., Doroski, M., Doty, J., Engtrakul, J.J., Harris, T.M., Huband, M., Knafels, J.D., Leach, K.L., Liu, S., Marfat, A., McAllister, L., McElroy, E., Menard, C.A., Mitton-Fry, M., Mullins, L., Noe, M.C., O'Donnell, J., Oliver, R., Penzien, J., Plummer, M., Shanmugasundaram, V., Thoma, C., Tomaras, A.P., Uccello, D.P., Vaz, A., Wishka, D.G.(2012) J Med Chem 55: 1662-1670
- PubMed: 22257165
- DOI: https://doi.org/10.1021/jm2014875
- Primary Citation of Related Structures:
3UHM - PubMed Abstract:
The synthesis and biological activity of a new series of LpxC inhibitors represented by pyridone methylsulfone hydroxamate 2a is presented. Members of this series have improved solubility and free fraction when compared to compounds in the previously described biphenyl methylsulfone hydroxamate series, and they maintain superior Gram-negative antibacterial activity to comparator agents.
- Worldwide Medicinal Chemistry, Pfizer Worldwide Research and Development, 445 Eastern Point Road, Groton, Connecticut 06340, United States. justin.montgomery@pfizer.com
Organizational Affiliation: