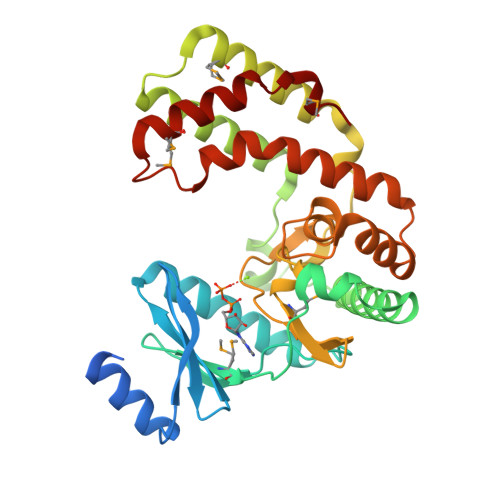
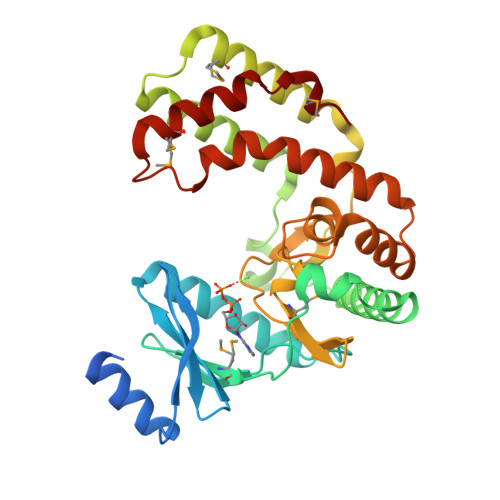
Crystal structure of aminoglycoside phosphotransferase APH(2'')-Ib, ADP-bound
Stogios, P.J., Minasov, G., Singer, A.U., Tan, K., Nocek, B., Evdokimova, E., Egorova, O., Di Leo, R., Savchenko, A., Anderson, W.F., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.