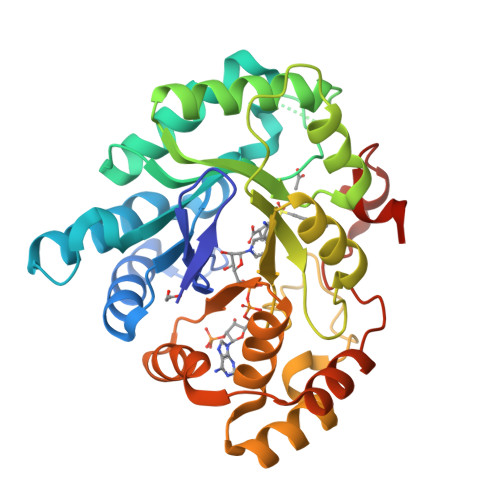
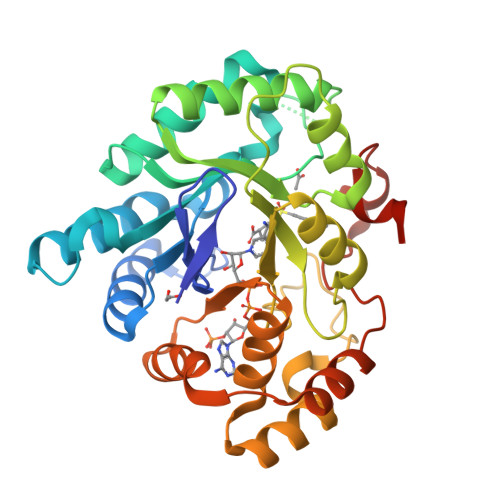
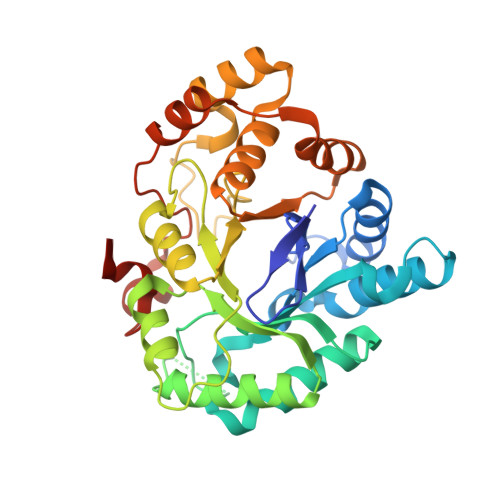
3-(3,4-Dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic acids; a New Class of Highly Potent and Selective Inhibitors of the Type 5 17-beta-hydroxysteroid Dehydrogenase AKR1C3
Jamieson, S.M., Brooke, D.G., Heinrich, D., Atwell, G.J., Silva, S., Hamilton, E.J., Turnbull, A.P., Rigoreau, L.J., Trivier, E., Soudy, C., Samlal, S.S., Owen, P.J., Schroeder, E., Raynham, T., Flanagan, J.U., Denny, W.A.(2012) J Med Chem 55: 7746-7758
- PubMed: 22877157
- DOI: https://doi.org/10.1021/jm3007867
- Primary Citation of Related Structures:
4FA3, 4FAL, 4FAM - PubMed Abstract:
A high-throughput screen identified 3-(3,4-dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic acid as a novel, highly potent (low nM), and isoform-selective (1500-fold) inhibitor of aldo-keto reductase AKR1C3: a target of interest in both breast and prostate cancer. Crystal structure studies showed that the carboxylate group occupies the oxyanion hole in the enzyme, while the sulfonamide provides the correct twist to allow the dihydroisoquinoline to bind in an adjacent hydrophobic pocket. SAR studies around this lead showed that the positioning of the carboxylate was critical, although it could be substituted by acid isosteres and amides. Small substituents on the dihydroisoquinoline gave improvements in potency. A set of "reverse sulfonamides" showed a 12-fold preference for the R stereoisomer. The compounds showed good cellular potency, as measured by inhibition of AKR1C3 metabolism of a known dinitrobenzamide substrate, with a broad rank order between enzymic and cellular activity, but amide analogues were more effective than predicted by the cellular assay.
Organizational Affiliation:
Auckland Cancer Society Research Centre, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand.