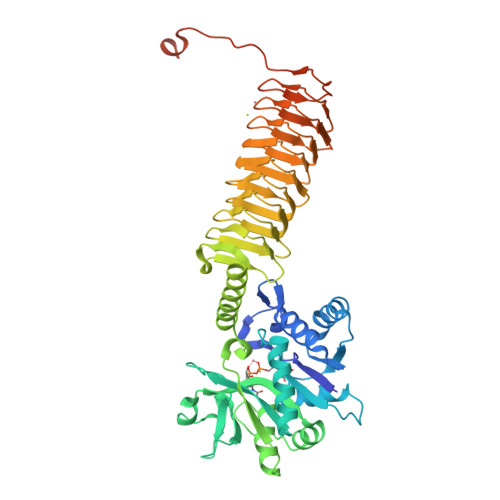
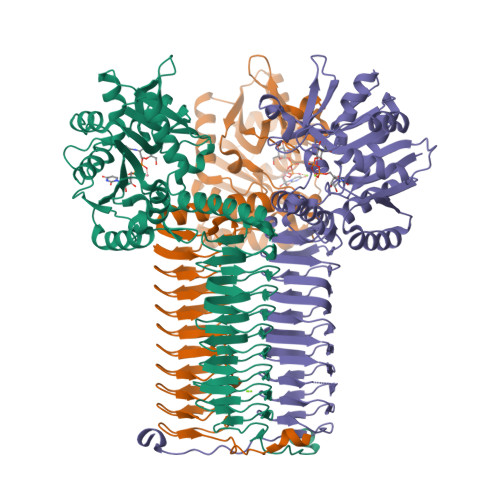
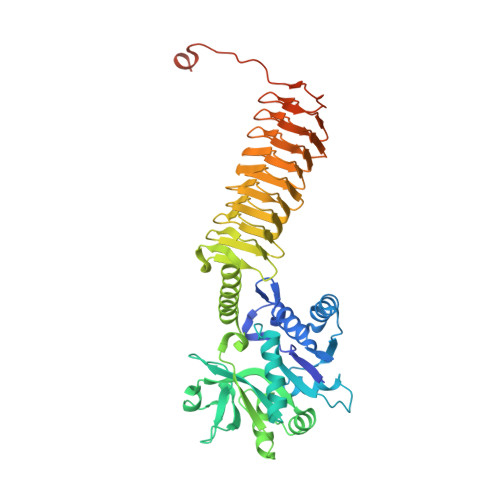
Crystal structures identify an atypical two-metal-ion mechanism for uridyltransfer in GlmU: its significance to sugar nucleotidyl transferases
Jagtap, P.K.A., Verma, S.K., Vithani, N., Bais, V.S., Prakash, B.(2013) J Mol Biology 425: 1745-1759
- PubMed: 23485416
- DOI: https://doi.org/10.1016/j.jmb.2013.02.019
- Primary Citation of Related Structures:
4G87, 4HCQ - PubMed Abstract:
N-Acetylglucosamine-1-phosphate uridyltransferase (GlmU), exclusive to prokaryotes, is a bifunctional enzyme that synthesizes UDP-GlcNAc-an important component of the cell wall of many microorganisms. Uridyltransfer, one of the reactions it catalyzes, involves binding GlcNAc-1-P, UTP and Mg(2+) ions; however, whether one or two ions catalyze this reaction remains ambiguous. Here, we resolve this using biochemical and crystallographic studies on GlmU from Mycobacterium tuberculosis (GlmU(Mtb)) and identify a two-metal-ion mechanism (mechanism-B). In contrast to well-established two-metal mechanism (mechanism-A) for enzymes acting on nucleic acids, mechanism-B is distinct in the way the two Mg(2+) ions (Mg(2+)A and Mg(2+)B) are positioned and stabilized. Further, attempts to delineate the roles of the metal ions in substrate stabilization, nucleophile activation and transition-state stabilization are presented. Interestingly, a detailed analysis of the available structures of sugar nucleotidyl transferases (SNTs) suggests that they too would utilize mechanism-B rather than mechanism-A. Based on this, SNTs could be classified into Group-I, which employs the two-metal mechanism-B as in GlmU, and Group-II that employs a variant one-metal mechanism-B, wherein the role of Mg(2+)A is substituted by a conserved lysine. Strikingly, eukaryotic SNTs appear confined to Group-II. Recognizing these differences may be important in the design of selective inhibitors against microbial nucleotidyl transferases.
Organizational Affiliation:
Department of Biological Sciences and Bioengineering, Indian Institute of Technology, Kanpur 208016, India.