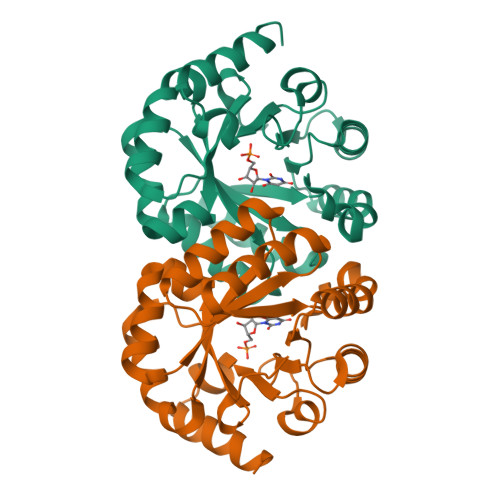
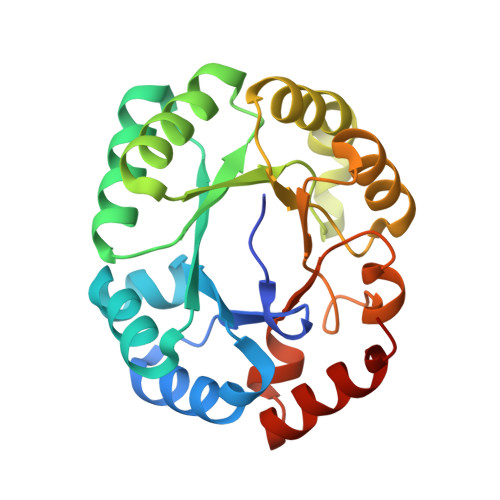
Conformational changes in orotidine 5'-monophosphate decarboxylase: a structure-based explanation for how the 5'-phosphate group activates the enzyme.
Desai, B.J., Wood, B.M., Fedorov, A.A., Fedorov, E.V., Goryanova, B., Amyes, T.L., Richard, J.P., Almo, S.C., Gerlt, J.A.(2012) Biochemistry 51: 8665-8678
- PubMed: 23030629
- DOI: https://doi.org/10.1021/bi301188k
- Primary Citation of Related Structures:
3LI0, 3P5Y, 3P5Z, 3P60, 3P61, 3QEZ, 3QF0, 3QMR, 3QMS, 3QMT, 3RLU, 3RLV, 3SJ3, 3V1P, 4FX6, 4FX8, 4FXR, 4GC4 - PubMed Abstract:
The binding of a ligand to orotidine 5'-monophosphate decarboxylase (OMPDC) is accompanied by a conformational change from an open, inactive conformation (E(o)) to a closed, active conformation (E(c)). As the substrate traverses the reaction coordinate to form the stabilized vinyl carbanion/carbene intermediate, interactions that destabilize the carboxylate group of the substrate and stabilize the intermediate (in the E(c)·S(‡) complex) are enforced. Focusing on the OMPDC from Methanothermobacter thermautotrophicus, we find the "remote" 5'-phosphate group of the substrate activates the enzyme 2.4 × 10(8)-fold; the activation is equivalently described by an intrinsic binding energy (IBE) of 11.4 kcal/mol. We studied residues in the activation that (1) directly contact the 5'-phosphate group, (2) participate in a hydrophobic cluster near the base of the active site loop that sequesters the bound substrate from the solvent, and (3) form hydrogen bonding interactions across the interface between the "mobile" and "fixed" half-barrel domains of the (β/α)(8)-barrel structure. Our data support a model in which the IBE provided by the 5'-phosphate group is used to allow interactions both near the N-terminus of the active site loop and across the domain interface that stabilize both the E(c)·S and E(c)·S(‡) complexes relative to the E(o)·S complex. The conclusion that the IBE of the 5'-phosphate group provides stabilization to both the E(c)·S and E(c)·S(‡) complexes, not just the E(c)·S(‡) complex, is central to understanding the structural origins of enzymatic catalysis as well as the requirements for the de novo design of enzymes that catalyze novel reactions.
Organizational Affiliation:
Departments of Biochemistry and Chemistry, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.