One enzyme, many reactions: structural basis for the various reactions catalyzed by naphthalene 1,2-dioxygenase.
Ferraro, D.J., Okerlund, A., Brown, E., Ramaswamy, S.(2017) IUCrJ 4: 648-656
- PubMed: 28989720
- DOI: https://doi.org/10.1107/S2052252517008223
- Primary Citation of Related Structures:
4HJL, 4HKV, 4HM0, 4HM2, 4HM3, 4HM4, 4HM5, 4HM6, 4HM7, 4HM8 - PubMed Abstract:
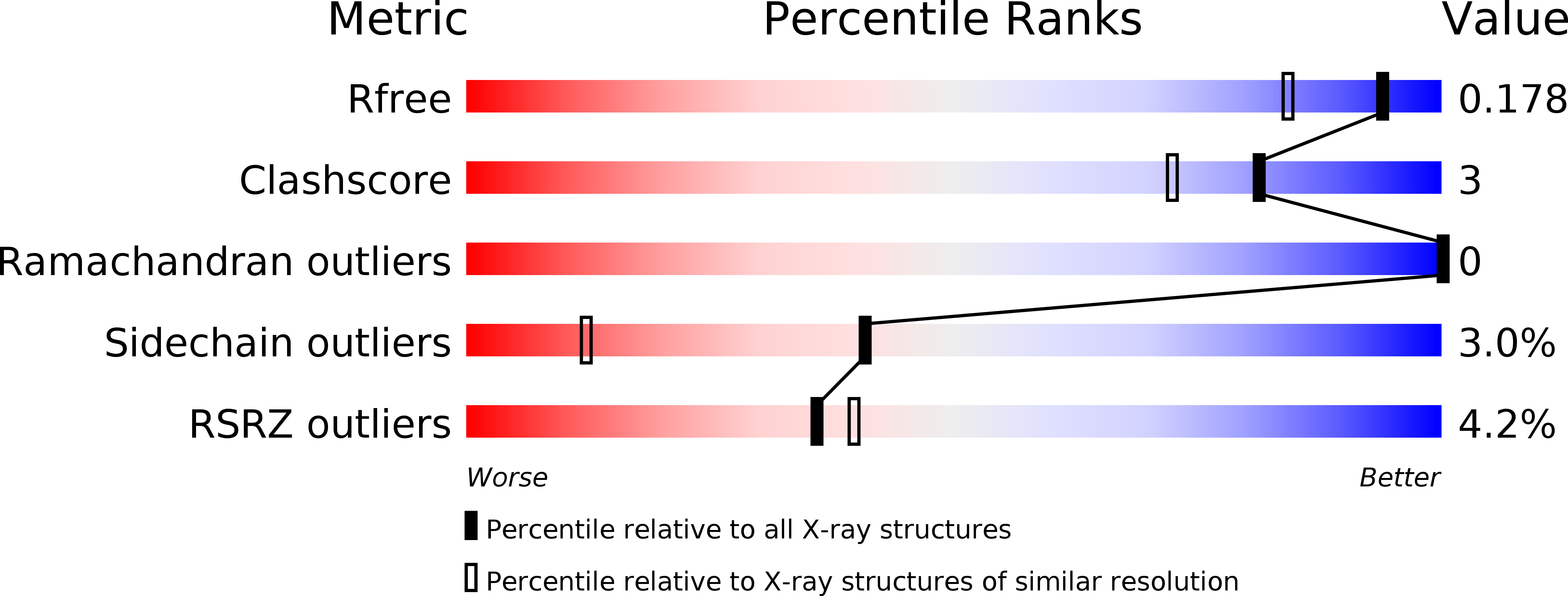
Rieske nonheme iron oxygenases (ROs) are a well studied class of enzymes. Naphthalene 1,2-dioxygenase (NDO) is used as a model to study ROs. Previous work has shown how side-on binding of oxygen to the mononuclear iron provides this enzyme with the ability to catalyze stereospecific and regiospecific cis -dihydroxylation reactions. It has been well documented that ROs catalyze a variety of other reactions, including mono-oxygenation, desaturation, O- and N-dealkylation, sulfoxidation etc . NDO itself catalyzes a variety of these reactions. Structures of NDO in complex with a number of different substrates show that the orientation of the substrate in the active site controls not only the regiospecificity and stereospecificity, but also the type of reaction catalyzed. It is proposed that the mononuclear iron-activated dioxygen attacks the atoms of the substrate that are most proximal to it. The promiscuity of delivering two products (apparently by two different reactions) from the same substrate can be explained by the possible binding of the substrate in slightly different orientations aided by the observed flexibility of residues in the binding pocket.
Organizational Affiliation:
Department of Biochemistry, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.