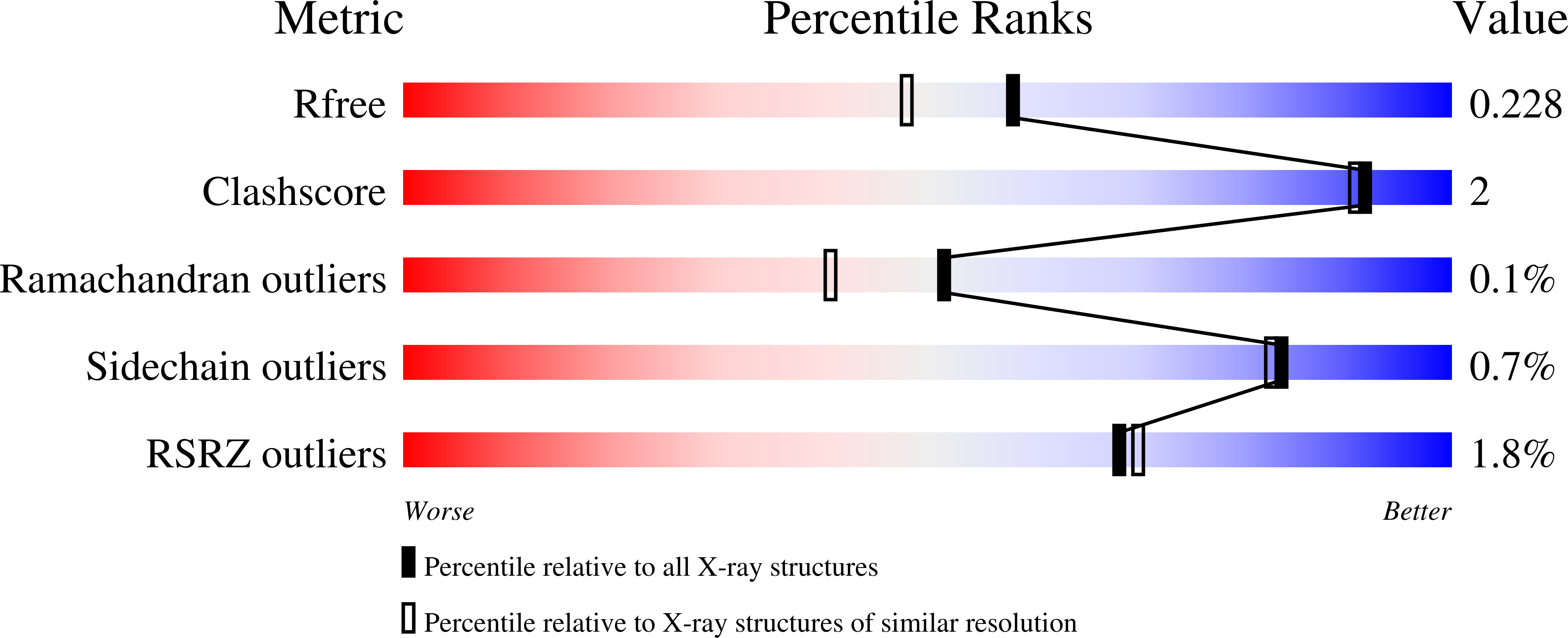
alpha-L-fucosidase inhibition by pyrrolidine-ferrocene hybrids: rationalization of ligand-binding properties by structural studies.
Hottin, A., Wright, D.W., Steenackers, A., Delannoy, P., Dubar, F., Biot, C., Davies, G.J., Behr, J.B.(2013) Chemistry 19: 9526-9533
- PubMed: 23740878
- DOI: https://doi.org/10.1002/chem.201301001
- Primary Citation of Related Structures:
4JFS, 4JFT, 4JFU, 4JFV, 4JFW - PubMed Abstract:
Enhanced metabolism of fucose through fucosidase overexpression is a signature of some cancer types, thus suggesting that fucosidase-targetted ligands could play the role of drug-delivery vectors. Herein, we describe the synthesis of a new series of pyrrolidine-ferrocene conjugates, consisting of a L-fuco-configured dihydroxypyrrolidine as the fucosidase ligand armed with a cytotoxic ferrocenylamine moeity. Three-dimensional structures of several of these fucosidase inhibitors reveal transition-state-mimicking (3)E conformations. Elaboration with the ferrocenyl moiety results in sub-micromolar inhibitors of both bovine and bacterial fucosidases, with the 3D structure of the latter revealing electron density indicative of highly mobile alkylferrocene compounds. The best compounds show a strong antiproliferative effect, with up to 100% inhibition of the proliferation of MDA-MB-231 cancer cells at 50 μM.
Organizational Affiliation:
Université de Reims Champagne-Ardenne, Institut de Chimie Moléculaire de Reims, CNRS UMR 7312, UFR des Sciences Exactes et Naturelles, 51687 Reims Cedex 2, France.