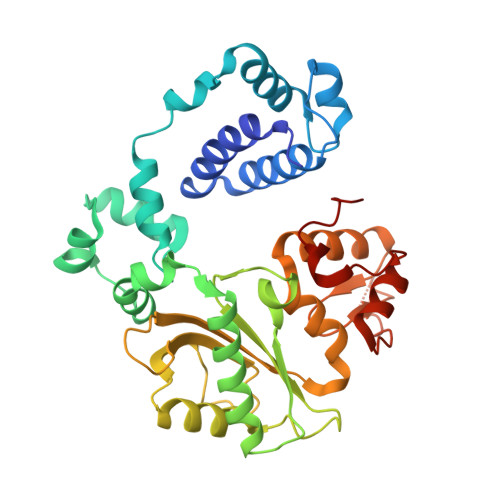
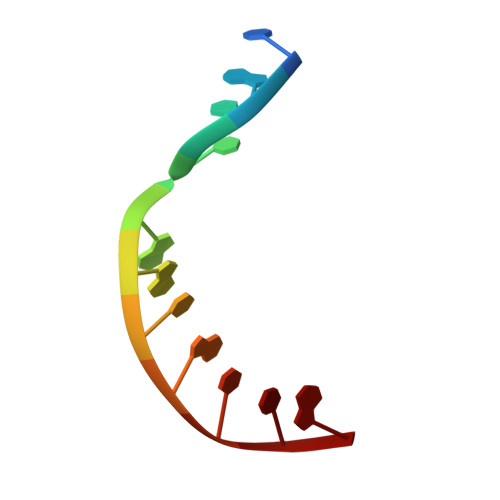
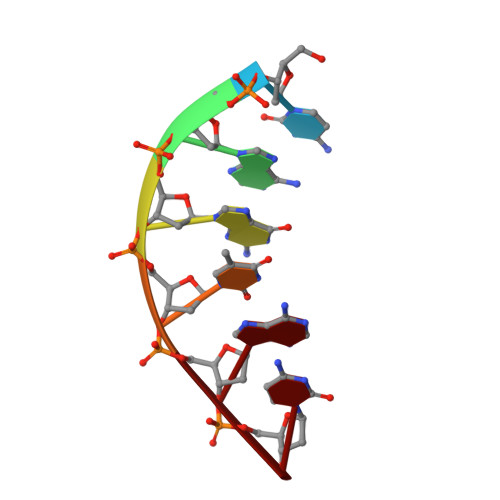
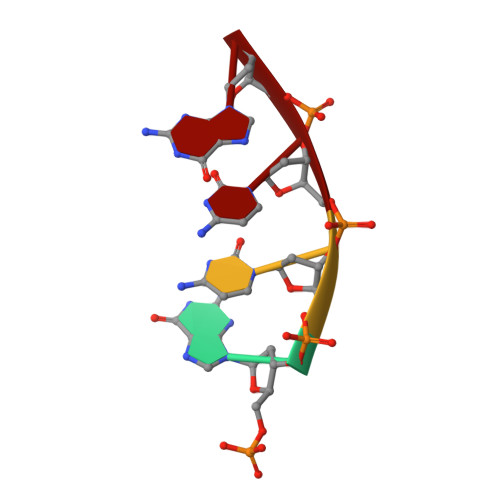
Structural basis for the binding and incorporation of nucleotide analogs with L-stereochemistry by human DNA polymerase lambda.
Vyas, R., Zahurancik, W.J., Suo, Z.(2014) Proc Natl Acad Sci U S A 111: E3033-E3042
- PubMed: 25015085
- DOI: https://doi.org/10.1073/pnas.1401286111
- PubMed Abstract:
Although lamivudine and emtricitabine, two L-deoxycytidine analogs, have been widely used as antiviral drugs for years, a structural basis for D-stereoselectivity against L-dNTPs, enantiomers of natural nucleotides (D-dNTPs), by any DNA polymerase or reverse transcriptase has not been established due to lack of a ternary structure of a polymerase, DNA, and an incoming L-dNTP. Here, we report 2.10-2.25 Å ternary crystal structures of human DNA polymerase λ, DNA, and L-deoxycytidine 5'-triphosphate (L-dCTP), or the triphosphates of lamivudine ((-)3TC-TP) and emtricitabine ((-)FTC-TP) with four ternary complexes per asymmetric unit. The structures of these 12 ternary complexes reveal that relative to D-deoxycytidine 5'-triphosphate (D-dCTP) in the canonical ternary structure of Polλ-DNA-D-dCTP, L-dCTP, (-)3TC-TP, and (-)FTC-TP all have their ribose rotated by 180°. Among the four ternary complexes with a specific L-nucleotide, two are similar and show that the L-nucleotide forms three Watson-Crick hydrogen bonds with the templating nucleotide dG and adopts a chair-like triphosphate conformation. In the remaining two similar ternary complexes, the L-nucleotide surprisingly interacts with the side chain of a conserved active site residue R517 through one or two hydrogen bonds, whereas the templating dG is anchored by a hydrogen bond with the side chain of a semiconserved residue Y505. Furthermore, the triphosphate of the L-nucleotide adopts an unprecedented N-shaped conformation. Our mutagenic and kinetic studies further demonstrate that the side chain of R517 is critical for the formation of the abovementioned four complexes along proposed catalytic pathways for L-nucleotide incorporation and provide the structural basis for the D-stereoselectivity of a DNA polymerase.
Organizational Affiliation:
Department of Chemistry and Biochemistry and.