CH-pi "T-Shape" Interaction with Histidine Explains Binding of Aromatic Galactosides to Pseudomonas aeruginosa Lectin LecA
Kadam, R.U., Garg, D., Schwartz, J., Visini, R., Sattler, M., Stocker, A., Darbre, T., Reymond, J.L.(2013) ACS Chem Biol 8: 1925-1930
- PubMed: 23869965
- DOI: https://doi.org/10.1021/cb400303w
- Primary Citation of Related Structures:
4LJH, 4LK6, 4LK7 - PubMed Abstract:
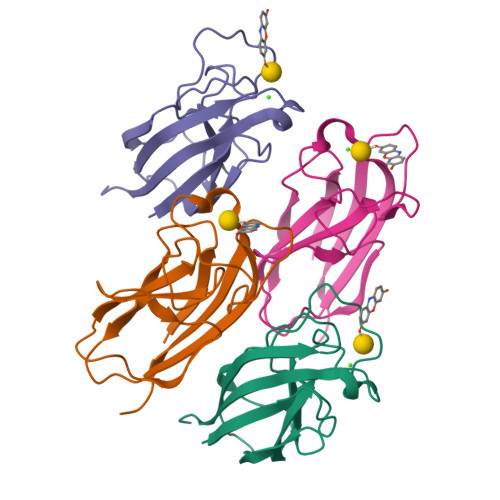
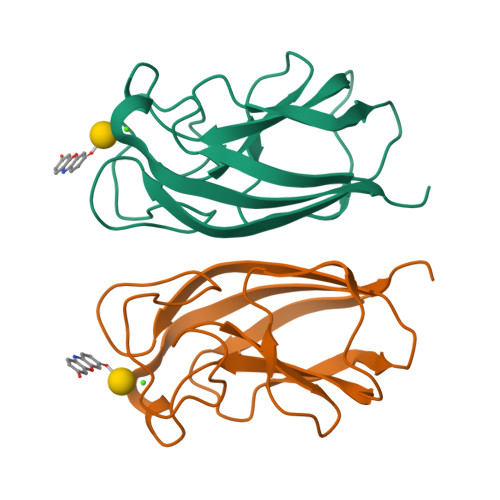
The galactose specific lectin LecA mediates biofilm formation in the opportunistic pathogen P. aeruginosa . The interaction between LecA and aromatic β-galactoside biofilm inhibitors involves an intermolecular CH-π T-shape interaction between C(ε1)-H of residue His50 in LecA and the aromatic ring of the galactoside aglycone. The generality of this interaction was tested in a diverse family of β-galactosides. LecA binding to aromatic β-galactosides (KD ∼ 8 μM) was consistently stronger than to aliphatic β-galactosides (KD ∼ 36 μM). The CH-π interaction was observed in the X-ray crystal structures of six different LecA complexes, with shorter than the van der Waals distances indicating productive binding. Related XH/cation/π-π interactions involving other residues were identified in complexes of aromatic glycosides with a variety of carbohydrate binding proteins such as concanavalin A. Exploiting such interactions might be generally useful in drug design against these targets.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Berne , Freiestrasse 3, 3012 Berne, Switzerland.