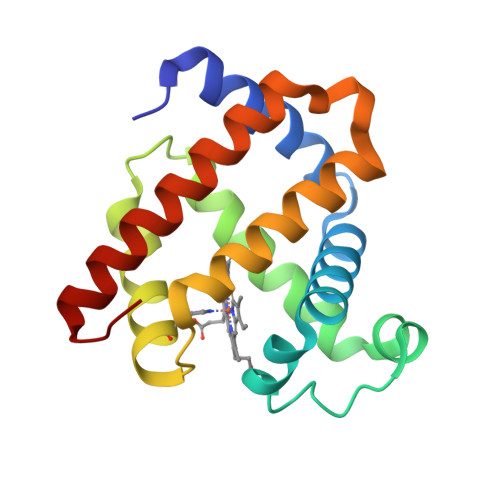
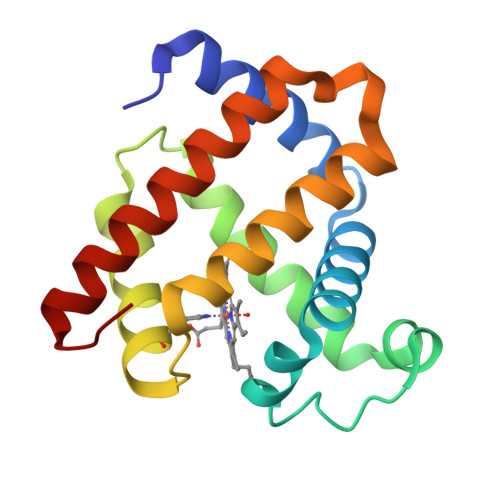
How a novel tyrosine-heme cross-link fine-tunes the structure and functions of heme proteins: a direct comparitive study of L29H/F43Y myoglobin
Yan, D.J., Yuan, H., Li, W., Xiang, Y., He, B., Nie, C.M., Wen, G.B., Lin, Y.W., Tan, X.(2015) Dalton Trans 44: 18815-18822
- PubMed: 26458300
- DOI: https://doi.org/10.1039/c5dt03040d
- Primary Citation of Related Structures:
4LPI, 5C6Y - PubMed Abstract:
A heme-protein cross-link is a key post-translational modification (PTM) of heme proteins. Meanwhile, the structural and functional consequences of heme-protein cross-links are not fully understood, due to limited studies on a direct comparison of the same protein with and without the cross-link. A Tyr-heme cross-link with a C-O bond is a newly discovered PTM of heme proteins, and is spontaneously formed in F43Y myoglobin (Mb) between the Tyr hydroxyl group and the heme 4-vinyl group in vivo. In this study, we found that with an additional distal His29 introduced in the heme pocket, the double mutant L29H/F43Y Mb can form two distinct forms under different protein purification conditions, with and without a novel Tyr-heme cross-link. By solving the X-ray structures of both forms of L29H/F43Y Mb and performing spectroscopic studies, we made a direct structural and functional comparison in the same protein scaffold. It revealed that the Tyr-heme cross-link regulates the heme distal hydrogen-bonding network, and fine-tunes not only the spectroscopic and ligand binding properties, but also the protein reactivity. Moreover, the formation of the Tyr-heme cross-link in the double mutant L29H/F43Y Mb was investigated in vitro. This study addressed the key issue of how Tyr-heme cross-link fine-tunes the structure and functions of the heme protein, and provided a plausible mechanism for the formation of the newly discovered Tyr-heme cross-link.
Organizational Affiliation:
School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China. linlinying@hotmail.com ywlin@usc.edu.cn.