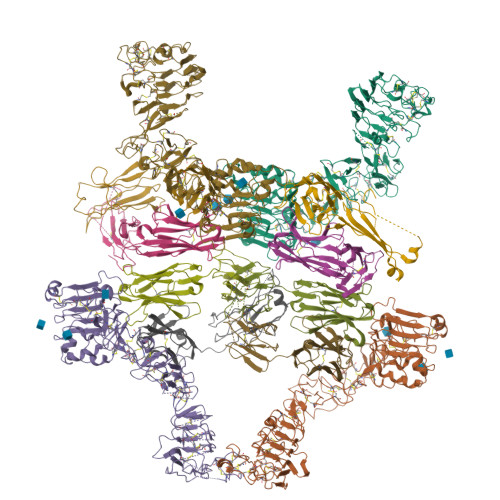
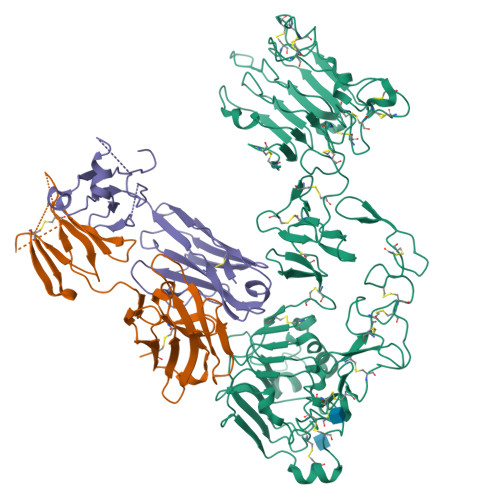
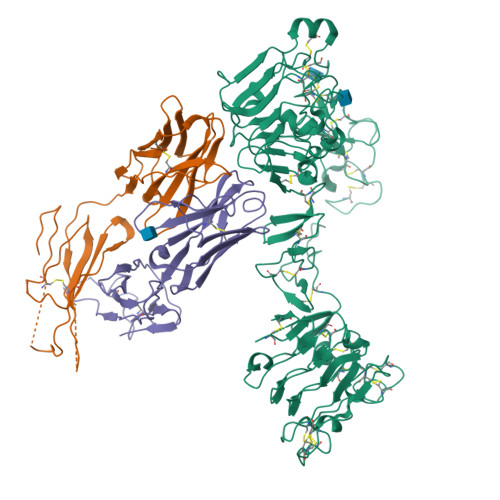
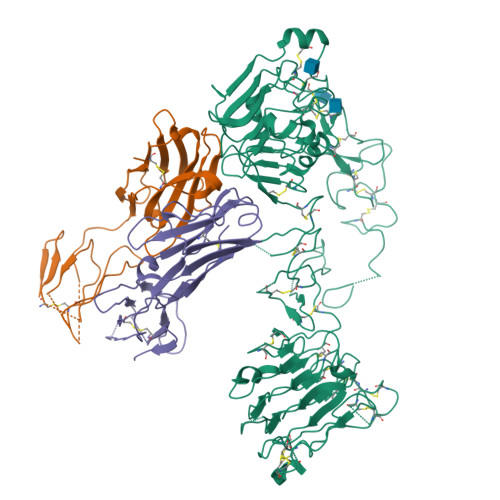
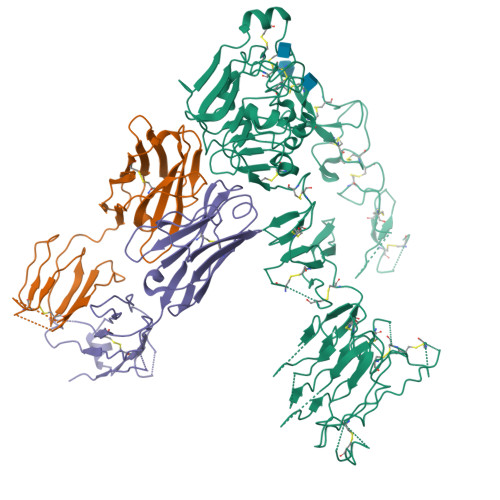
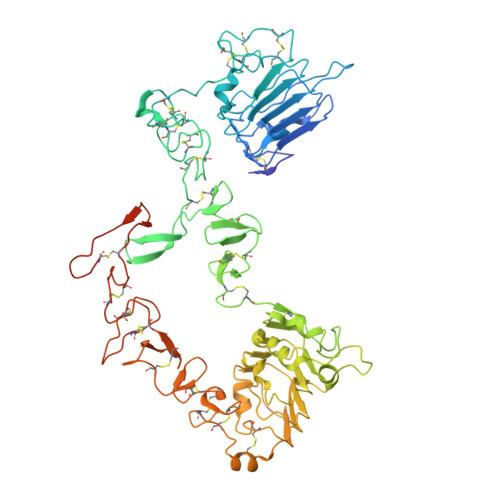
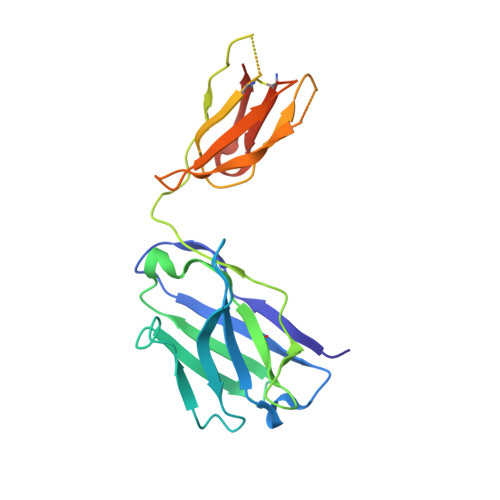
Inhibition of ErbB3 by a monoclonal antibody that locks the extracellular domain in an inactive configuration.
Lee, S., Greenlee, E.B., Amick, J.R., Ligon, G.F., Lillquist, J.S., Natoli, E.J., Hadari, Y., Alvarado, D., Schlessinger, J.(2015) Proc Natl Acad Sci U S A 112: 13225-13230
- PubMed: 26460020
- DOI: https://doi.org/10.1073/pnas.1518361112
- Primary Citation of Related Structures:
5CUS - PubMed Abstract:
ErbB3 (HER3) is a member of the EGF receptor (EGFR) family of receptor tyrosine kinases, which, unlike the other three family members, contains a pseudo kinase in place of a tyrosine kinase domain. In cancer, ErbB3 activation is driven by a ligand-dependent mechanism through the formation of heterodimers with EGFR, ErbB2, or ErbB4 or via a ligand-independent process through heterodimerization with ErbB2 overexpressed in breast tumors or other cancers. Here we describe the crystal structure of the Fab fragment of an antagonistic monoclonal antibody KTN3379, currently in clinical development in human cancer patients, in complex with the ErbB3 extracellular domain. The structure reveals a unique allosteric mechanism for inhibition of ligand-dependent or ligand-independent ErbB3-driven cancers by binding to an epitope that locks ErbB3 in an inactive conformation. Given the similarities in the mechanism of ErbB receptor family activation, these findings could facilitate structure-based design of antibodies that inhibit EGFR and ErbB4 by an allosteric mechanism.
Organizational Affiliation:
Department of Pharmacology, Yale School of Medicine, New Haven, CT 06520;