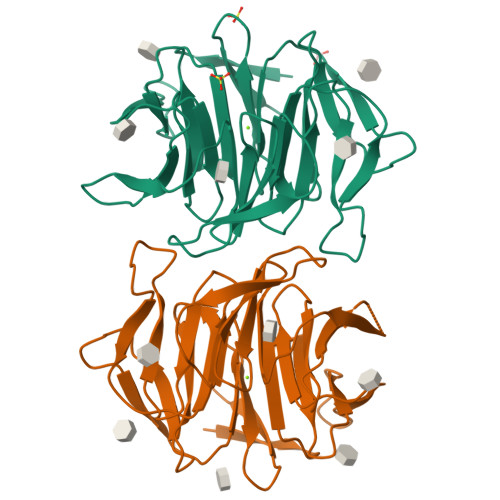
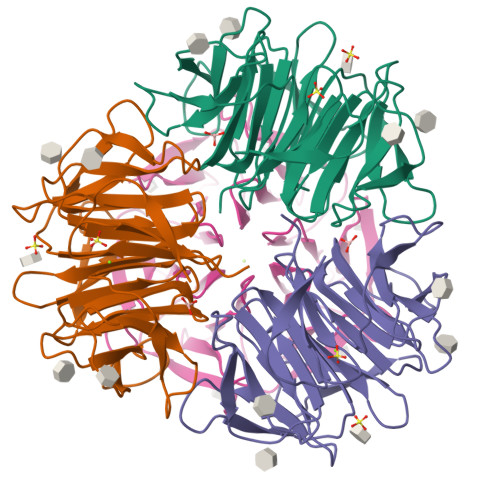
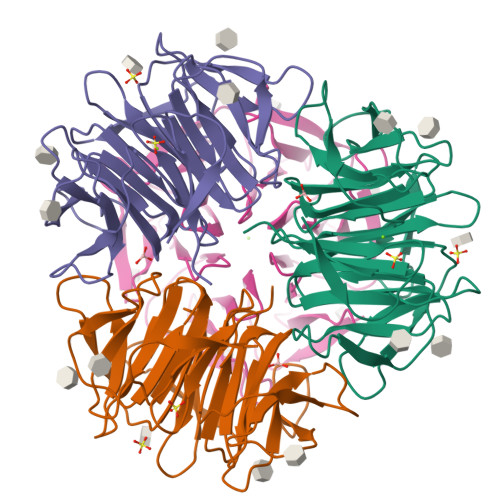
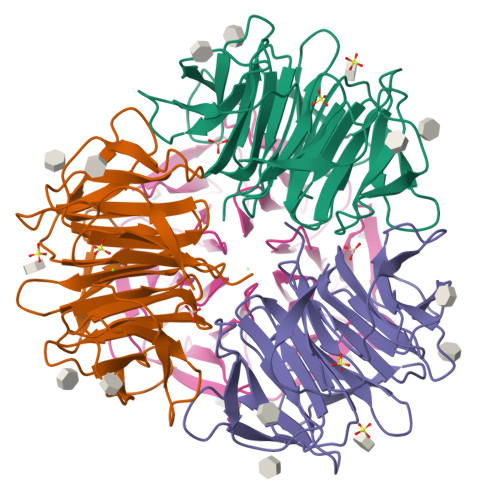
Crystal Structures of Fungal Tectonin in Complex with O-Methylated Glycans Suggest Key Role in Innate Immune Defense.
Sommer, R., Makshakova, O.N., Wohlschlager, T., Hutin, S., Marsh, M., Titz, A., Kunzler, M., Varrot, A.(2018) Structure 26: 391-402.e4
- PubMed: 29398527
- DOI: https://doi.org/10.1016/j.str.2018.01.003
- Primary Citation of Related Structures:
5FSB, 5FSC - PubMed Abstract:
Innate immunity is the first line of defense against pathogens and predators. To initiate a response, it relies on the detection of invaders, where lectin-carbohydrate interactions play a major role. O-Methylated glycans were previously identified as non-self epitopes and conserved targets for defense effector proteins belonging to the tectonin superfamily. Here, we present two crystal structures of Tectonin 2 from the mushroom Laccaria bicolor in complex with methylated ligands, unraveling the molecular basis for this original specificity. Furthermore, they revealed the formation of a ball-shaped tetramer with 24 binding sites distributed at its surface, resembling a small virus capsid. Based on the crystal structures, a methylation recognition motif was identified and found in the sequence of many tectonins from bacteria to human. Our results support a key role of tectonins in innate defense based on a distinctive and conserved type of lectin-glycan interaction.
Organizational Affiliation:
Chemical Biology of Carbohydrates, Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), 66123 Saarbrücken, Germany; Deutsches Zentrum für Infektionsforschung, Standort Hannover-Braunschweig, Hannover, Germany.