Discovery of GluN2A-Selective NMDA Receptor Positive Allosteric Modulators (PAMs): Tuning Deactivation Kinetics via Structure-Based Design.
Volgraf, M., Sellers, B.D., Jiang, Y., Wu, G., Ly, C.Q., Villemure, E., Pastor, R.M., Yuen, P.W., Lu, A., Luo, X., Liu, M., Zhang, S., Sun, L., Fu, Y., Lupardus, P.J., Wallweber, H.J., Liederer, B.M., Deshmukh, G., Plise, E., Tay, S., Reynen, P., Herrington, J., Gustafson, A., Liu, Y., Dirksen, A., Dietz, M.G., Liu, Y., Wang, T.M., Hanson, J.E., Hackos, D., Scearce-Levie, K., Schwarz, J.B.(2016) J Med Chem 59: 2760-2779
- PubMed: 26919761
- DOI: https://doi.org/10.1021/acs.jmedchem.5b02010
- Primary Citation of Related Structures:
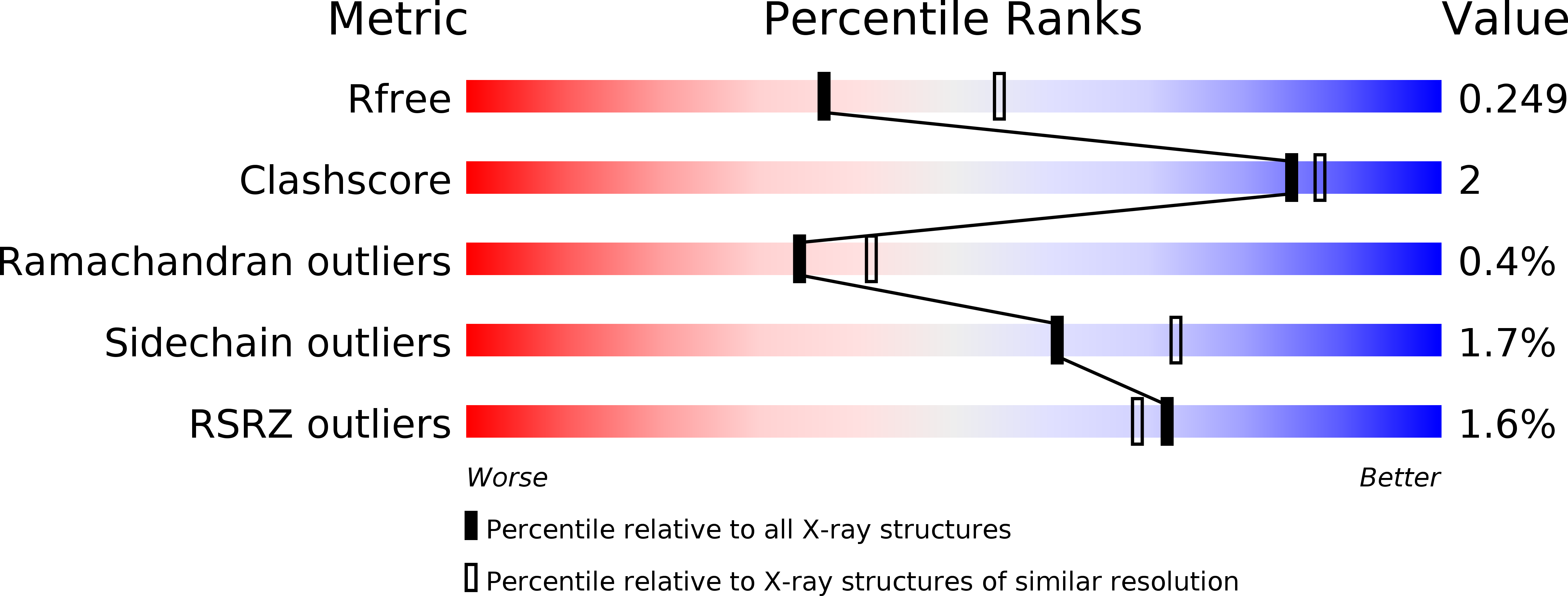
5I2K, 5I2N, 5KDT - PubMed Abstract:
The N-methyl-D-aspartate receptor (NMDAR) is a Na(+) and Ca(2+) permeable ionotropic glutamate receptor that is activated by the coagonists glycine and glutamate. NMDARs are critical to synaptic signaling and plasticity, and their dysfunction has been implicated in a number of neurological disorders, including schizophrenia, depression, and Alzheimer's disease. Herein we describe the discovery of potent GluN2A-selective NMDAR positive allosteric modulators (PAMs) starting from a high-throughput screening hit. Using structure-based design, we sought to increase potency at the GluN2A subtype, while improving selectivity against related α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). The structure-activity relationship of channel deactivation kinetics was studied using a combination of electrophysiology and protein crystallography. Effective incorporation of these strategies resulted in the discovery of GNE-0723 (46), a highly potent and brain penetrant GluN2A-selective NMDAR PAM suitable for in vivo characterization.
Organizational Affiliation:
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA, Beijing 100176, PR China.