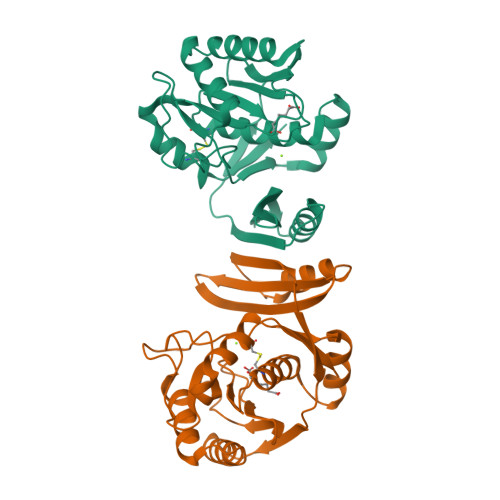
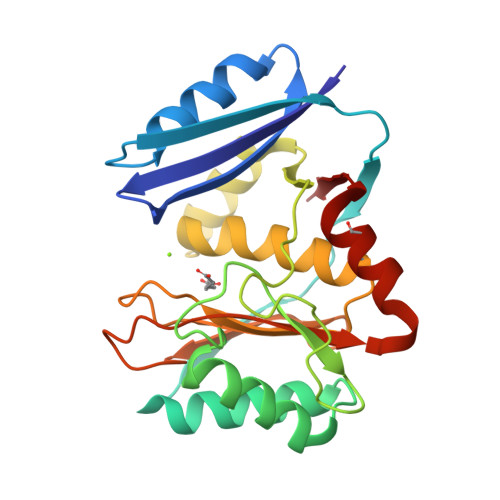
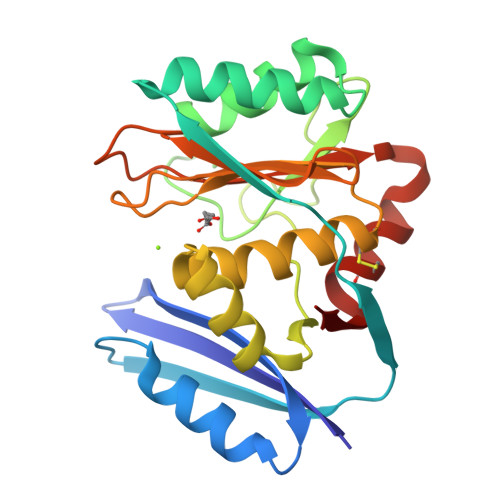
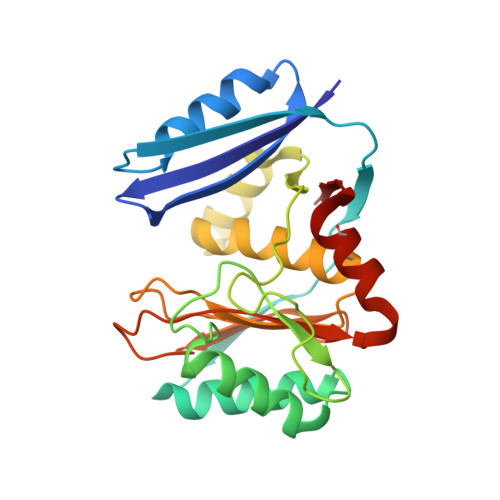
The pimeloyl-CoA synthetase BioW defines a new fold for adenylate-forming enzymes.
Estrada, P., Manandhar, M., Dong, S.H., Deveryshetty, J., Agarwal, V., Cronan, J.E., Nair, S.K.(2017) Nat Chem Biol 13: 668-674
- PubMed: 28414711
- DOI: https://doi.org/10.1038/nchembio.2359
- Primary Citation of Related Structures:
5TV5, 5TV6, 5TV8, 5TVA - PubMed Abstract:
Reactions that activate carboxylates through acyl-adenylate intermediates are found throughout biology and include acyl- and aryl-CoA synthetases and tRNA synthetases. Here we describe the characterization of Aquifex aeolicus BioW, which represents a new protein fold within the superfamily of adenylating enzymes. Substrate-bound structures identified the enzyme active site and elucidated the mechanistic strategy for conjugating CoA to the seven-carbon α,ω-dicarboxylate pimelate, a biotin precursor. Proper position of reactive groups for the two half-reactions is achieved solely through movements of active site residues, as confirmed by site-directed mutational analysis. The ability of BioW to hydrolyze adenylates of noncognate substrates is reminiscent of pre-transfer proofreading observed in some tRNA synthetases, and we show that this activity can be abolished by mutation of a single residue. These studies illustrate how BioW can carry out three different biologically prevalent chemical reactions (adenylation, thioesterification, and proofreading) in the context of a new protein fold.
Organizational Affiliation:
Department of Biochemistry, University of Illinois at Urbana-Champaign, Urbana, Illinois, USA.