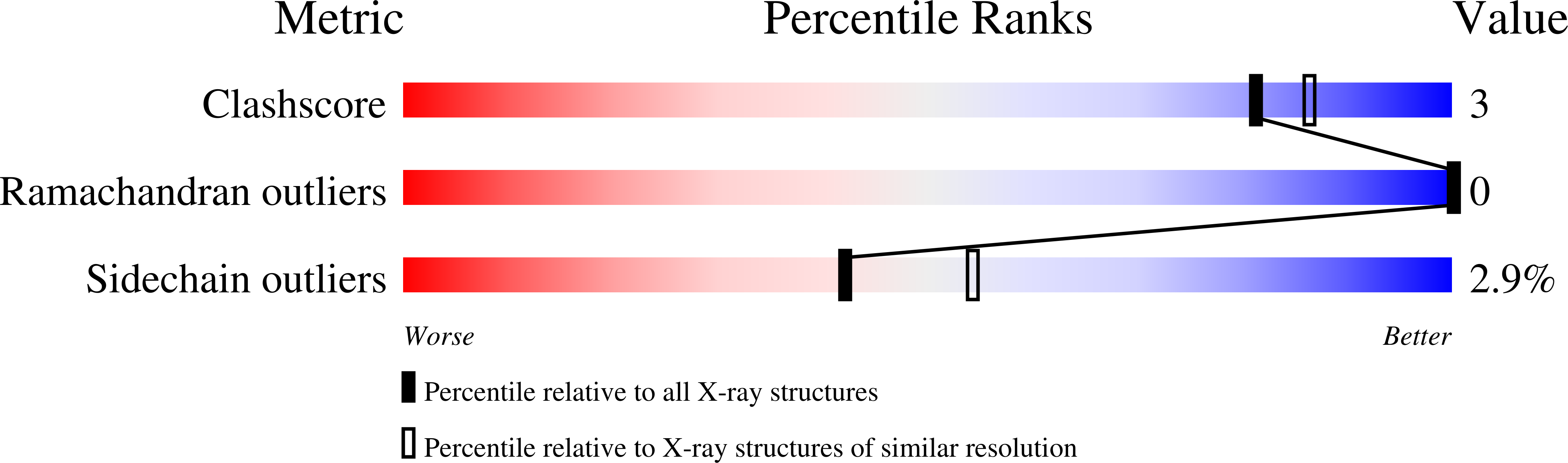Neutron crystallographic studies of T4 lysozyme at cryogenic temperature.
Li, L., Shukla, S., Meilleur, F., Standaert, R.F., Pierce, J., Myles, D.A.A., Cuneo, M.J.(2017) Protein Sci 26: 2098-2104
- PubMed: 28707382
- DOI: https://doi.org/10.1002/pro.3231
- Primary Citation of Related Structures:
5VNQ, 5VNR - PubMed Abstract:
Bacteriophage T4 lysozyme (T4L) has been used as a paradigm for seminal biophysical studies on protein structure, dynamics, and stability. Approximately 700 mutants of this protein and their respective complexes have been characterized by X-ray crystallography; however, despite the high resolution diffraction limits attained in several studies, no hydrogen atoms were reported being visualized in the electron density maps. To address this, a 2.2 Å-resolution neutron data set was collected at 80 K from a crystal of perdeuterated T4L pseudo-wild type. We describe a near complete atomic structure of T4L, which includes the positions of 1737 hydrogen atoms determined by neutron crystallography. The cryogenic neutron model reveals explicit detail of the hydrogen bonding interactions in the protein, in addition to the protonation states of several important residues.
Organizational Affiliation:
Oak Ridge National Laboratory, Neutron Sciences Directorate, Oak Ridge, Tennessee, 37831.