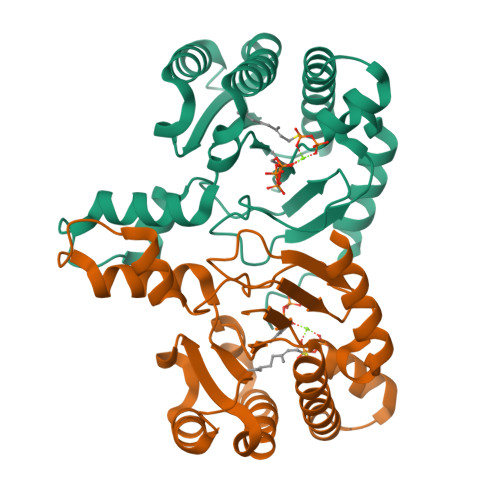
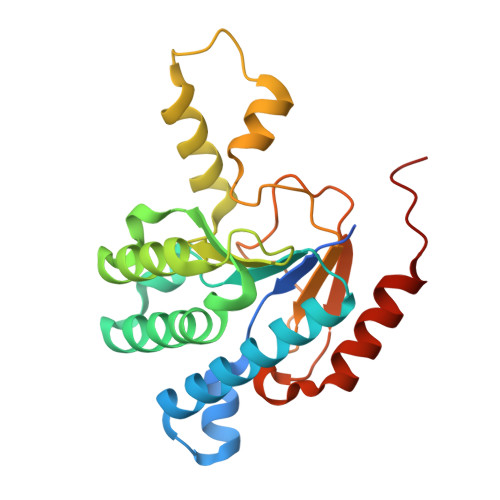
"Head-to-Middle" and "Head-to-Tail" cis-Prenyl Transferases: Structure of Isosesquilavandulyl Diphosphate Synthase.
Gao, J., Ko, T.P., Chen, L., Malwal, S.R., Zhang, J., Hu, X., Qu, F., Liu, W., Huang, J.W., Cheng, Y.S., Chen, C.C., Yang, Y., Zhang, Y., Oldfield, E., Guo, R.T.(2018) Angew Chem Int Ed Engl 57: 683-687
- PubMed: 29215779
- DOI: https://doi.org/10.1002/anie.201710185
- Primary Citation of Related Structures:
5XK3, 5XK6, 5XK7, 5XK8, 5XK9 - PubMed Abstract:
We report the first X-ray crystallographic structure of the "head-to-middle" prenyltransferase, isosesquilavandulyl diphosphate synthase, involved in biosynthesis of the merochlorin class of antibiotics. The protein adopts the ζ or cis-prenyl transferase fold but remarkably, unlike tuberculosinol adenosine synthase and other cis-prenyl transferases (e.g. cis-farnesyl, decaprenyl, undecaprenyl diphosphate synthases), the large, hydrophobic side chain does not occupy a central hydrophobic tunnel. Instead, it occupies a surface pocket oriented at 90° to the hydrophobic tunnel. Product chain-length control is achieved by squeezing out the ligand from the conventional allylic S1 binding site, with proton abstraction being achieved using a diphosphate-Asn-Ser relay. The structures revise and unify our thinking as to the mechanism of action of many other prenyl transferases and may also be of use in engineering new merochlorin-class antibiotics.
Organizational Affiliation:
Industrial Enzymes National Engineering Laboratory, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin, 300308, China.