Identification of a CARM1 Inhibitor with Potent In Vitro and In Vivo Activity in Preclinical Models of Multiple Myeloma.
Drew, A.E., Moradei, O., Jacques, S.L., Rioux, N., Boriack-Sjodin, A.P., Allain, C., Scott, M.P., Jin, L., Raimondi, A., Handler, J.L., Ott, H.M., Kruger, R.G., McCabe, M.T., Sneeringer, C., Riera, T., Shapiro, G., Waters, N.J., Mitchell, L.H., Duncan, K.W., Moyer, M.P., Copeland, R.A., Smith, J., Chesworth, R., Ribich, S.A.(2017) Sci Rep 7: 17993-17993
- PubMed: 29269946
- DOI: https://doi.org/10.1038/s41598-017-18446-z
- Primary Citation of Related Structures:
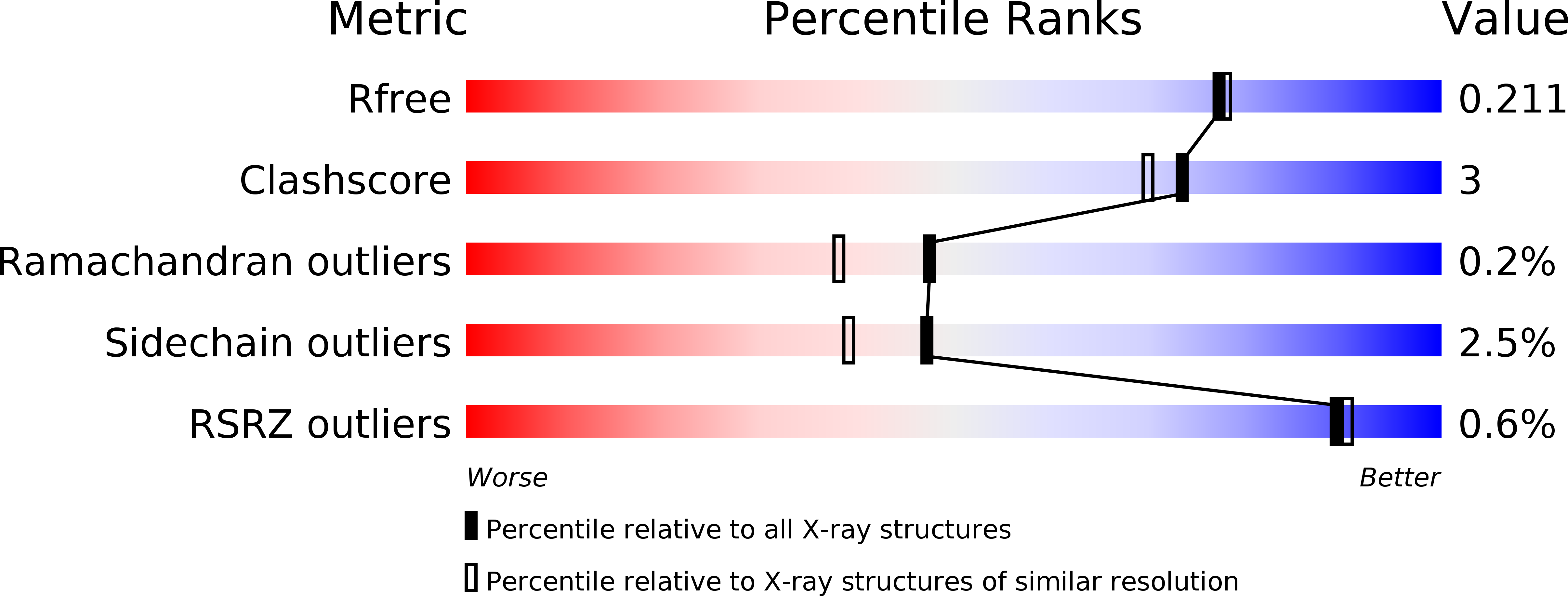
6ARJ, 6ARV - PubMed Abstract:
CARM1 is an arginine methyltransferase with diverse histone and non-histone substrates implicated in the regulation of cellular processes including transcriptional co-activation and RNA processing. CARM1 overexpression has been reported in multiple cancer types and has been shown to modulate oncogenic pathways in in vitro studies. Detailed understanding of the mechanism of action of CARM1 in oncogenesis has been limited by a lack of selective tool compounds, particularly for in vivo studies. We describe the identification and characterization of, to our knowledge, the first potent and selective inhibitor of CARM1 that exhibits anti-proliferative effects both in vitro and in vivo and, to our knowledge, the first demonstration of a role for CARM1 in multiple myeloma (MM). EZM2302 (GSK3359088) is an inhibitor of CARM1 enzymatic activity in biochemical assays (IC 50 = 6 nM) with broad selectivity against other histone methyltransferases. Treatment of MM cell lines with EZM2302 leads to inhibition of PABP1 and SMB methylation and cell stasis with IC 50 values in the nanomolar range. Oral dosing of EZM2302 demonstrates dose-dependent in vivo CARM1 inhibition and anti-tumor activity in an MM xenograft model. EZM2302 is a validated chemical probe suitable for further understanding the biological role CARM1 plays in cancer and other diseases.
Organizational Affiliation:
Epizyme, Inc., Cambridge, Massachusetts, USA. adrew@epizyme.com.