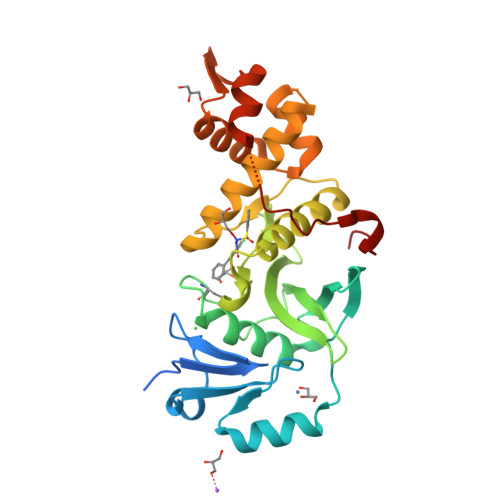
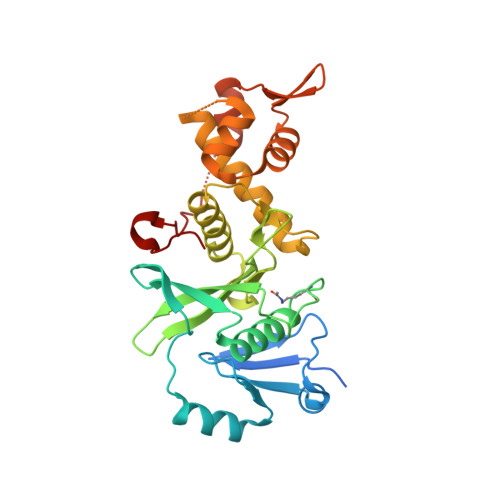
Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth.
Baell, J.B., Leaver, D.J., Hermans, S.J., Kelly, G.L., Brennan, M.S., Downer, N.L., Nguyen, N., Wichmann, J., McRae, H.M., Yang, Y., Cleary, B., Lagiakos, H.R., Mieruszynski, S., Pacini, G., Vanyai, H.K., Bergamasco, M.I., May, R.E., Davey, B.K., Morgan, K.J., Sealey, A.J., Wang, B., Zamudio, N., Wilcox, S., Garnham, A.L., Sheikh, B.N., Aubrey, B.J., Doggett, K., Chung, M.C., de Silva, M., Bentley, J., Pilling, P., Hattarki, M., Dolezal, O., Dennis, M.L., Falk, H., Ren, B., Charman, S.A., White, K.L., Rautela, J., Newbold, A., Hawkins, E.D., Johnstone, R.W., Huntington, N.D., Peat, T.S., Heath, J.K., Strasser, A., Parker, M.W., Smyth, G.K., Street, I.P., Monahan, B.J., Voss, A.K., Thomas, T.(2018) Nature 560: 253-257
- PubMed: 30069049
- DOI: https://doi.org/10.1038/s41586-018-0387-5
- Primary Citation of Related Structures:
6BA2, 6BA4, 6CT2 - PubMed Abstract:
Acetylation of histones by lysine acetyltransferases (KATs) is essential for chromatin organization and function 1 . Among the genes coding for the MYST family of KATs (KAT5-KAT8) are the oncogenes KAT6A (also known as MOZ) and KAT6B (also known as MORF and QKF) 2,3 . KAT6A has essential roles in normal haematopoietic stem cells 4-6 and is the target of recurrent chromosomal translocations, causing acute myeloid leukaemia 7,8 . Similarly, chromosomal translocations in KAT6B have been identified in diverse cancers 8 . KAT6A suppresses cellular senescence through the regulation of suppressors of the CDKN2A locus 9,10 , a function that requires its KAT activity 10 . Loss of one allele of KAT6A extends the median survival of mice with MYC-induced lymphoma from 105 to 413 days 11 . These findings suggest that inhibition of KAT6A and KAT6B may provide a therapeutic benefit in cancer. Here we present highly potent, selective inhibitors of KAT6A and KAT6B, denoted WM-8014 and WM-1119. Biochemical and structural studies demonstrate that these compounds are reversible competitors of acetyl coenzyme A and inhibit MYST-catalysed histone acetylation. WM-8014 and WM-1119 induce cell cycle exit and cellular senescence without causing DNA damage. Senescence is INK4A/ARF-dependent and is accompanied by changes in gene expression that are typical of loss of KAT6A function. WM-8014 potentiates oncogene-induced senescence in vitro and in a zebrafish model of hepatocellular carcinoma. WM-1119, which has increased bioavailability, arrests the progression of lymphoma in mice. We anticipate that this class of inhibitors will help to accelerate the development of therapeutics that target gene transcription regulated by histone acetylation.
Organizational Affiliation:
Medicinal Chemistry Theme, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia. Jonathan.Baell@monash.edu.