Structure of xyloglucan xylosyltransferase 1 reveals simple steric rules that define biological patterns of xyloglucan polymers.
Culbertson, A.T., Ehrlich, J.J., Choe, J.Y., Honzatko, R.B., Zabotina, O.A.(2018) Proc Natl Acad Sci U S A 115: 6064-6069
- PubMed: 29784804
- DOI: https://doi.org/10.1073/pnas.1801105115
- Primary Citation of Related Structures:
6BSU, 6BSV, 6BSW - PubMed Abstract:
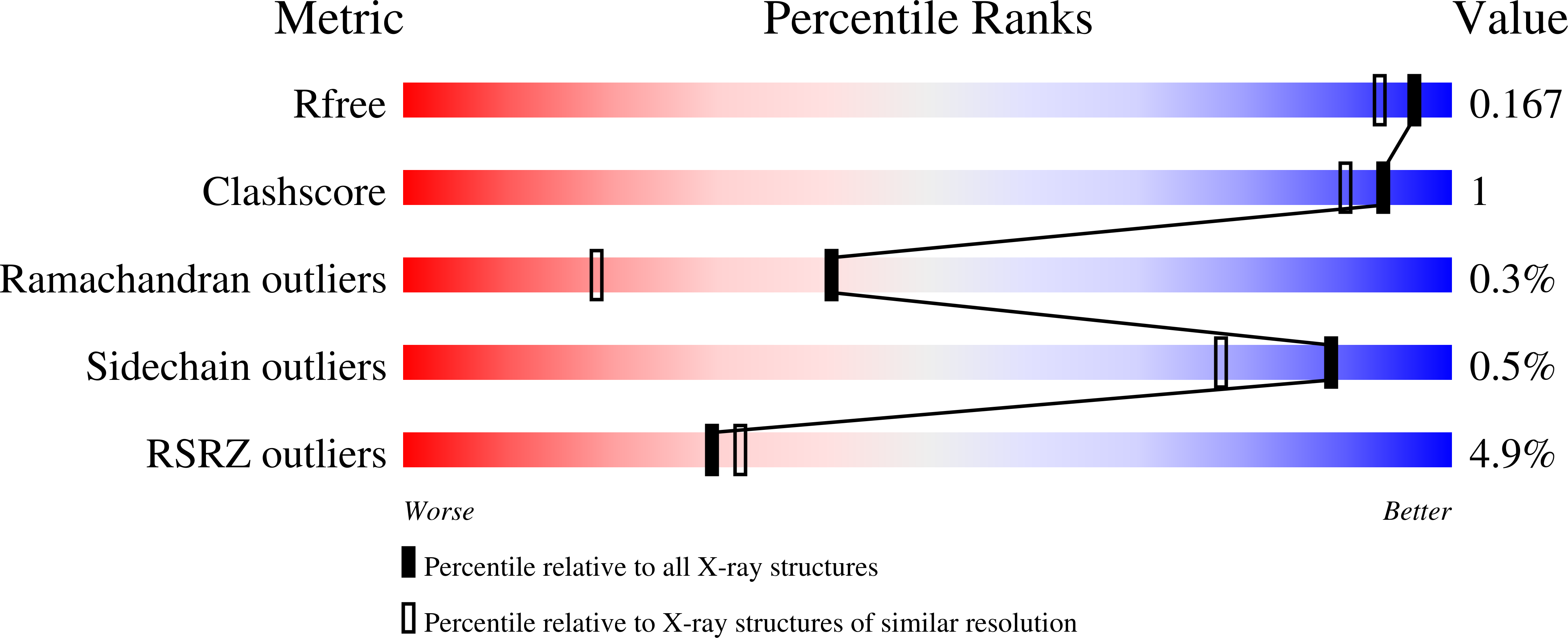
The plant cell wall is primarily a polysaccharide mesh of the most abundant biopolymers on earth. Although one of the richest sources of biorenewable materials, the biosynthesis of the plant polysaccharides is poorly understood. Structures of many essential plant glycosyltransferases are unknown and suitable substrates are often unavailable for in vitro analysis. The dearth of such information impedes the development of plants better suited for industrial applications. Presented here are structures of Arabidopsis xyloglucan xylosyltransferase 1 (XXT1) without ligands and in complexes with UDP and cellohexaose. XXT1 initiates side-chain extensions from a linear glucan polymer by transferring the xylosyl group from UDP-xylose during xyloglucan biosynthesis. XXT1, a homodimer and member of the GT-A fold family of glycosyltransferases, binds UDP analogously to other GT-A fold enzymes. Structures here and the properties of mutant XXT1s are consistent with a SN i -like catalytic mechanism. Distinct from other systems is the recognition of cellohexaose by way of an extended cleft. The XXT1 dimer alone cannot produce xylosylation patterns observed for native xyloglucans because of steric constraints imposed by the acceptor binding cleft. Homology modeling of XXT2 and XXT5, the other two xylosyltransferases involved in xyloglucan biosynthesis, reveals a structurally altered cleft in XXT5 that could accommodate a partially xylosylated glucan chain produced by XXT1 and/or XXT2. An assembly of the three XXTs can produce the xylosylation patterns of native xyloglucans, suggesting the involvement of an organized multienzyme complex in the xyloglucan biosynthesis.
Organizational Affiliation:
Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology, Iowa State University, Ames, IA 50011.