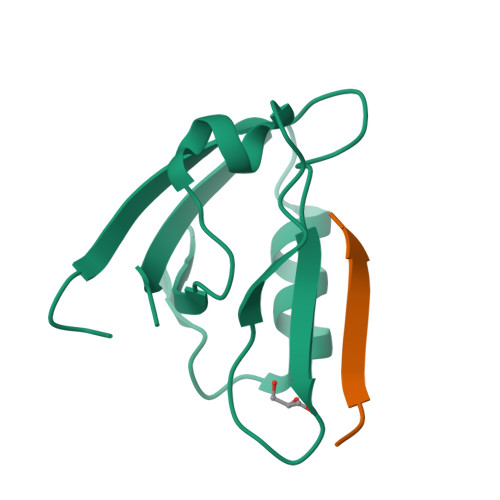
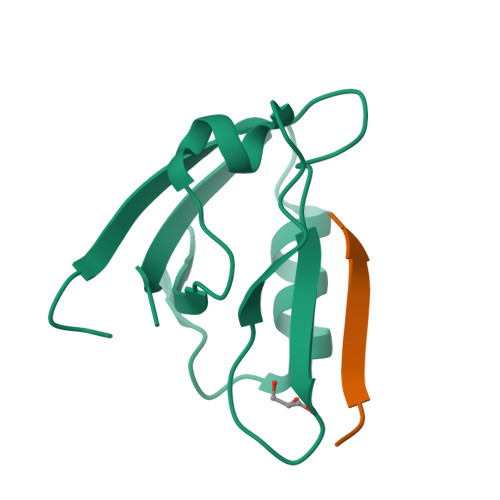
Probing the Architecture of a Multi-PDZ Domain Protein: Structure of PDZK1 in Solution.
Hajizadeh, N.R., Pieprzyk, J., Skopintsev, P., Flayhan, A., Svergun, D.I., Low, C.(2018) Structure 26: 1522-1533.e5
- PubMed: 30220543
- DOI: https://doi.org/10.1016/j.str.2018.07.016
- Primary Citation of Related Structures:
6EZI - PubMed Abstract:
The scaffolding protein PDZK1 has been associated with the regulation of membrane transporters. It contains four conserved PDZ domains, which typically recognize a 3-5-residue long motif at the C terminus of the binding partner. The atomic structures of the individual domains are available but their spatial arrangement in the full-length context influencing the binding properties remained elusive. Here we report a systematic study of full-length PDZK1 and deletion constructs using small-angle X-ray scattering, complemented with biochemical and functional studies on PDZK1 binding to known membrane protein partners. A hybrid modeling approach utilizing multiple scattering datasets yielded a well-defined, extended, asymmetric L-shaped domain organization of PDZK1 in contrast to a flexible "beads-on-string" model predicted by bioinformatics analysis. The linker regions of PDZK1 appear to play a central role in the arrangement of the four domains underlying the importance of studying scaffolding proteins in their full-length context.
Organizational Affiliation:
European Molecular Biology Laboratory, Hamburg Outstation, Building 25a, Notkestrasse 85, Hamburg 22607, Germany.