NVP-BHG712: Effects of Regioisomers on the Affinity and Selectivity toward the EPHrin Family.
Troster, A., Heinzlmeir, S., Berger, B.T., Gande, S.L., Saxena, K., Sreeramulu, S., Linhard, V., Nasiri, A.H., Bolte, M., Muller, S., Kuster, B., Medard, G., Kudlinzki, D., Schwalbe, H.(2018) ChemMedChem 13: 1629-1633
- PubMed: 29928781
- DOI: https://doi.org/10.1002/cmdc.201800398
- Primary Citation of Related Structures:
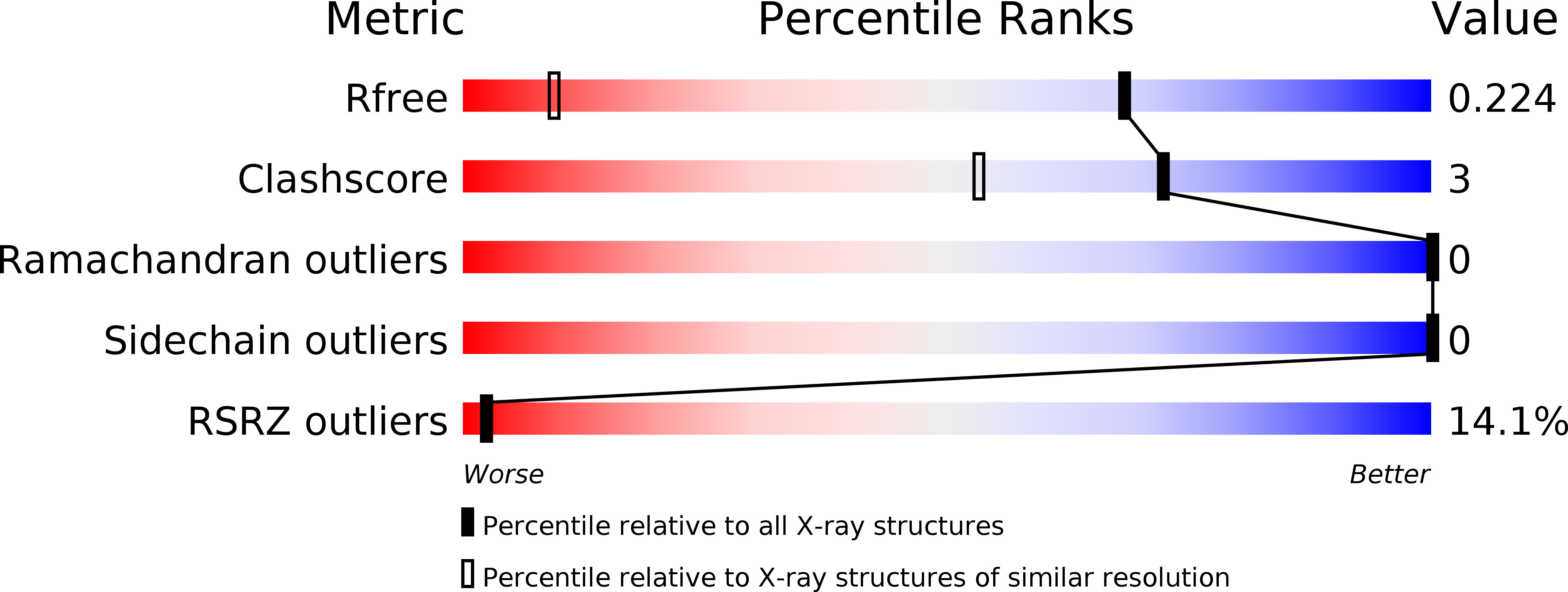
6FNF, 6FNG, 6FNH, 6FNI, 6FNJ, 6FNK, 6FNL, 6FNM - PubMed Abstract:
Erythropoietin-producing hepatocellular (EPH) receptors are transmembrane receptor tyrosine kinases. Their extracellular domains bind specifically to ephrin A/B ligands, and this binding modulates intracellular kinase activity. EPHs are key players in bidirectional intercellular signaling, controlling cell morphology, adhesion, and migration. They are increasingly recognized as cancer drug targets. We analyzed the binding of NVP-BHG712 (NVP) to EPHA2 and EPHB4. Unexpectedly, all tested commercially available NVP samples turned out to be a regioisomer (NVPiso) of the inhibitor, initially described in a Novartis patent application. They only differ by the localization of a single methyl group on either one of two adjacent nitrogen atoms. The two compounds of identical mass revealed different binding modes. Furthermore, both in vitro and in vivo experiments showed that the isomers differ in their kinase affinity and selectivity.
Organizational Affiliation:
Center for Biomolecular Magnetic Resonance (BMRZ), Institute for Organic Chemistry and Chemical Biology, Johann Wolfgang Goethe University, Max-von-Laue-Straße 7, 60438, Frankfurt am Main, Germany.