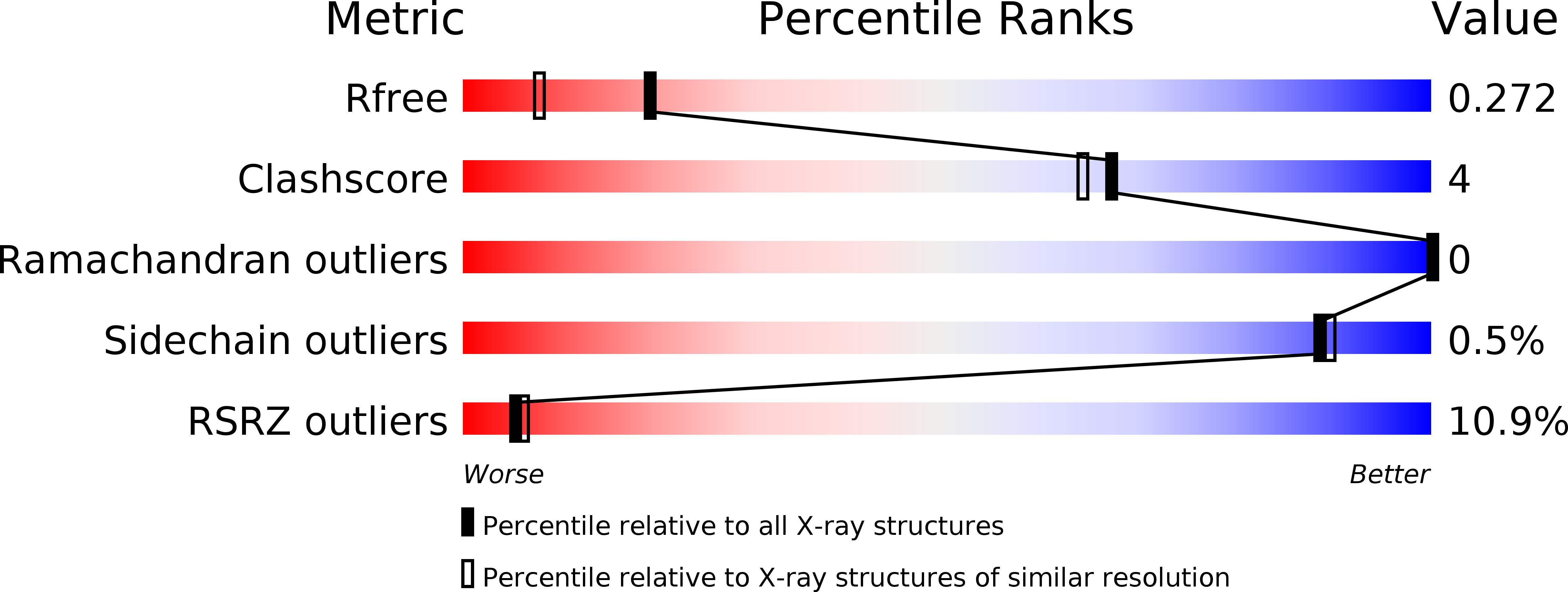
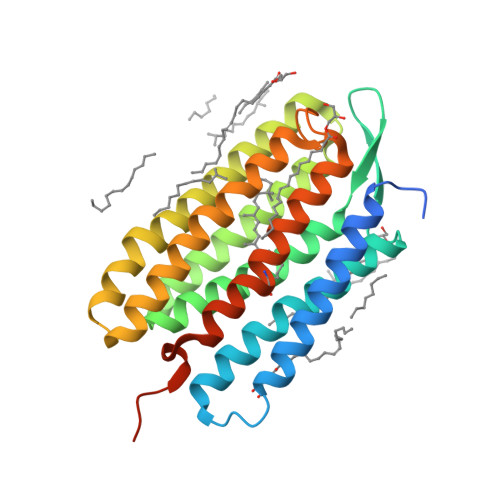
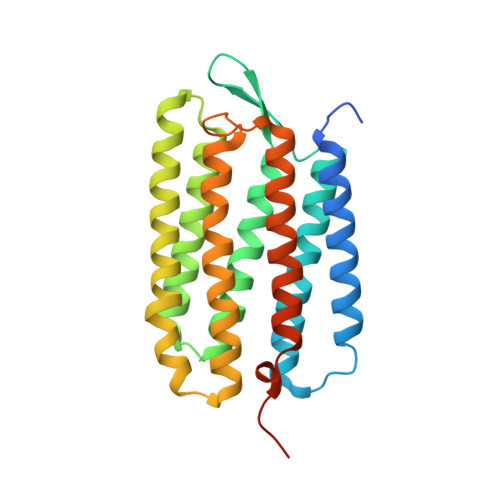
Retinal isomerization in bacteriorhodopsin captured by a femtosecond x-ray laser.
Nogly, P., Weinert, T., James, D., Carbajo, S., Ozerov, D., Furrer, A., Gashi, D., Borin, V., Skopintsev, P., Jaeger, K., Nass, K., Bath, P., Bosman, R., Koglin, J., Seaberg, M., Lane, T., Kekilli, D., Brunle, S., Tanaka, T., Wu, W., Milne, C., White, T., Barty, A., Weierstall, U., Panneels, V., Nango, E., Iwata, S., Hunter, M., Schapiro, I., Schertler, G., Neutze, R., Standfuss, J.(2018) Science 361
- PubMed: 29903883
- DOI: https://doi.org/10.1126/science.aat0094
- Primary Citation of Related Structures:
6G7H, 6G7I, 6G7J, 6G7K, 6G7L - PubMed Abstract:
Ultrafast isomerization of retinal is the primary step in photoresponsive biological functions including vision in humans and ion transport across bacterial membranes. We used an x-ray laser to study the subpicosecond structural dynamics of retinal isomerization in the light-driven proton pump bacteriorhodopsin. A series of structural snapshots with near-atomic spatial resolution and temporal resolution in the femtosecond regime show how the excited all-trans retinal samples conformational states within the protein binding pocket before passing through a twisted geometry and emerging in the 13-cis conformation. Our findings suggest ultrafast collective motions of aspartic acid residues and functional water molecules in the proximity of the retinal Schiff base as a key facet of this stereoselective and efficient photochemical reaction.
Organizational Affiliation:
Division of Biology and Chemistry-Laboratory for Biomolecular Research, Paul Scherrer Institut, 5232 Villigen, Switzerland.