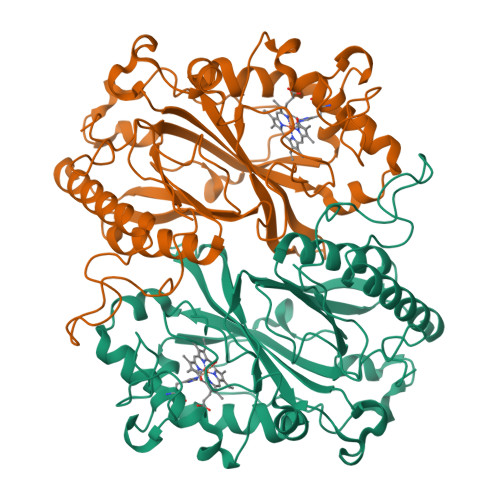
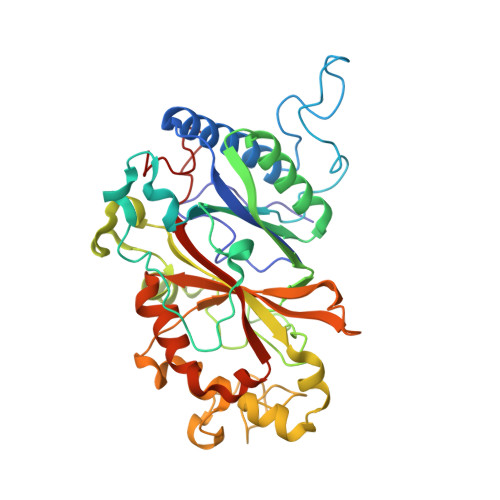
Dose-resolved serial synchrotron and XFEL structures of radiation-sensitive metalloproteins.
Ebrahim, A., Moreno-Chicano, T., Appleby, M.V., Chaplin, A.K., Beale, J.H., Sherrell, D.A., Duyvesteyn, H.M.E., Owada, S., Tono, K., Sugimoto, H., Strange, R.W., Worrall, J.A.R., Axford, D., Owen, R.L., Hough, M.A.(2019) IUCrJ 6: 543-551
- PubMed: 31316799
- DOI: https://doi.org/10.1107/S2052252519003956
- Primary Citation of Related Structures:
6I43, 6I7Z, 6I8E, 6I8I, 6I8J, 6I8K, 6I8O, 6I8P, 6I8Q, 6IBN, 6Q31, 6Q34, 6Q3D, 6Q3E - PubMed Abstract:
An approach is demonstrated to obtain, in a sample- and time-efficient manner, multiple dose-resolved crystal structures from room-temperature protein microcrystals using identical fixed-target supports at both synchrotrons and X-ray free-electron lasers (XFELs). This approach allows direct comparison of dose-resolved serial synchrotron and damage-free XFEL serial femtosecond crystallography structures of radiation-sensitive proteins. Specifically, serial synchrotron structures of a heme peroxidase enzyme reveal that X-ray induced changes occur at far lower doses than those at which diffraction quality is compromised (the Garman limit), consistent with previous studies on the reduction of heme proteins by low X-ray doses. In these structures, a functionally relevant bond length is shown to vary rapidly as a function of absorbed dose, with all room-temperature synchrotron structures exhibiting linear deformation of the active site compared with the XFEL structure. It is demonstrated that extrapolation of dose-dependent synchrotron structures to zero dose can closely approximate the damage-free XFEL structure. This approach is widely applicable to any protein where the crystal structure is altered by the synchrotron X-ray beam and provides a solution to the urgent requirement to determine intact structures of such proteins in a high-throughput and accessible manner.
Organizational Affiliation:
School of Biological Sciences, University of Essex, Wivenhoe Park, Colchester CO4 3SQ, UK.