Discovery of the Oral Leukotriene C4 Synthase Inhibitor (1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-fluoro-2-methylpropyl)amino]-3-methoxypyrazin-2-yl}carbonyl)cyclopropanecarboxylic Acid (AZD9898) as a New Treatment for Asthma.
Munck Af Rosenschold, M., Johannesson, P., Nikitidis, A., Tyrchan, C., Chang, H.F., Ronn, R., Chapman, D., Ullah, V., Nikitidis, G., Glader, P., Kack, H., Bonn, B., Wagberg, F., Bjorkstrand, E., Andersson, U., Swedin, L., Rohman, M., Andreasson, T., Bergstrom, E.L., Jiang, F., Zhou, X.H., Lundqvist, A.J., Malmberg, A., Ek, M., Gordon, E., Pettersen, A., Ripa, L., Davis, A.M.(2019) J Med Chem 62: 7769-7787
- PubMed: 31415176
- DOI: https://doi.org/10.1021/acs.jmedchem.9b00555
- Primary Citation of Related Structures:
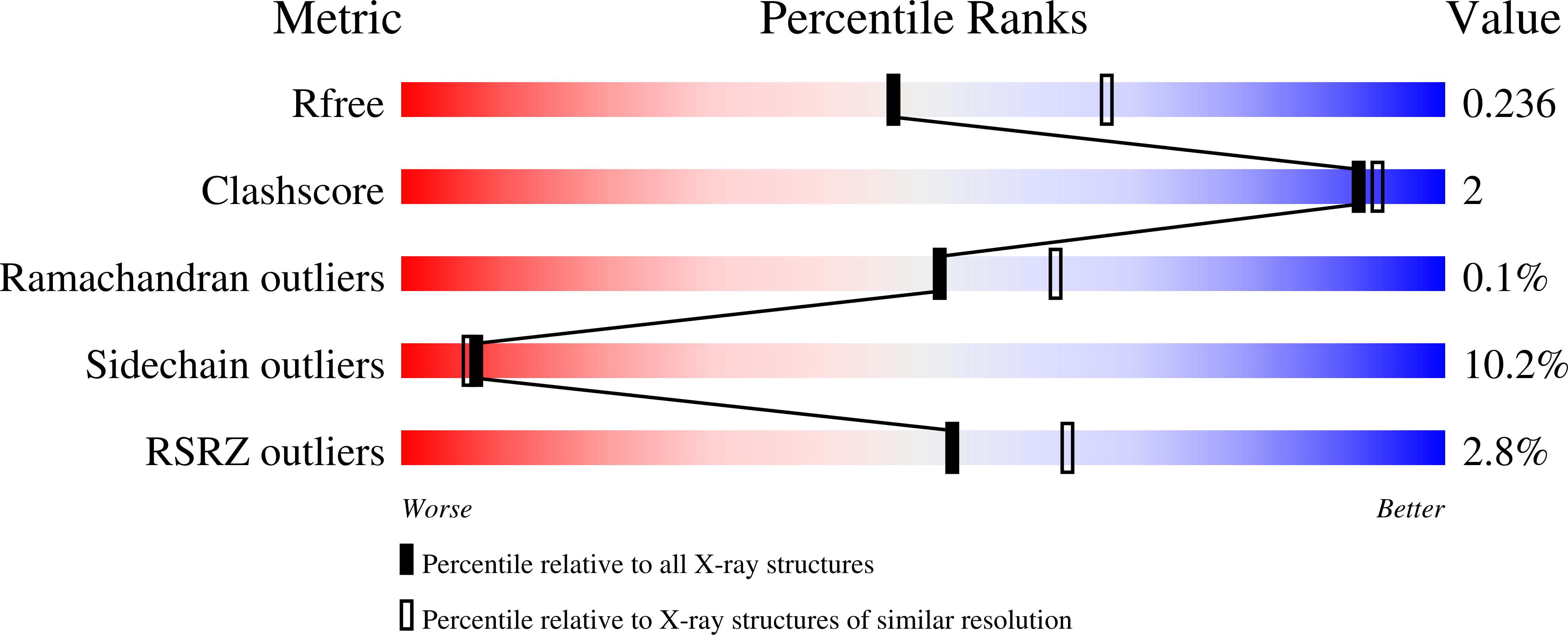
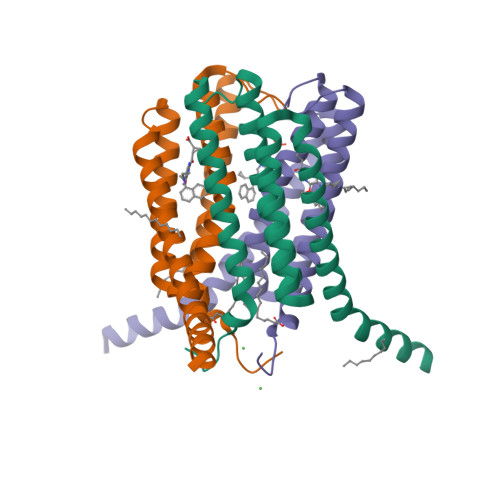
6R7D - PubMed Abstract:
While bronchodilators and inhaled corticosteroids are the mainstay of asthma treatment, up to 50% of asthmatics remain uncontrolled. Many studies show that the cysteinyl leukotriene cascade remains highly activated in some asthmatics, even those on high-dose inhaled or oral corticosteroids. Hence, inhibition of the leukotriene C4 synthase (LTC4S) enzyme could provide a new and differentiated core treatment for patients with a highly activated cysteinyl leukotriene cascade. Starting from a screening hit ( 3 ), a program to discover oral inhibitors of LTC4S led to (1 S ,2 S )-2-({5-[(5-chloro-2,4-difluorophenyl)(2-fluoro-2-methylpropyl)amino]-3-methoxypyrazin-2-yl}carbonyl)cyclopropanecarboxylic acid (AZD9898) ( 36 ), a picomolar LTC4S inhibitor (IC 50 = 0.28 nM) with high lipophilic ligand efficiency (LLE = 8.5), which displays nanomolar potency in cells (peripheral blood mononuclear cell, IC 50,free = 6.2 nM) and good in vivo pharmacodynamics in a calcium ionophore-stimulated rat model after oral dosing (in vivo, IC 50,free = 34 nM). Compound 36 mitigates the GABA binding, hepatic toxicity signal, and in vivo toxicology findings of an early lead compound 7 with a human dose predicted to be 30 mg once daily.
Organizational Affiliation:
Orexo AB , Virdings allé 32A , SE-75450 Uppsala , Sweden.