Structural characterization of the microbial enzyme urocanate reductase mediating imidazole propionate production.
Venskutonyte, R., Koh, A., Stenstrom, O., Khan, M.T., Lundqvist, A., Akke, M., Backhed, F., Lindkvist-Petersson, K.(2021) Nat Commun 12: 1347-1347
- PubMed: 33649331
- DOI: https://doi.org/10.1038/s41467-021-21548-y
- Primary Citation of Related Structures:
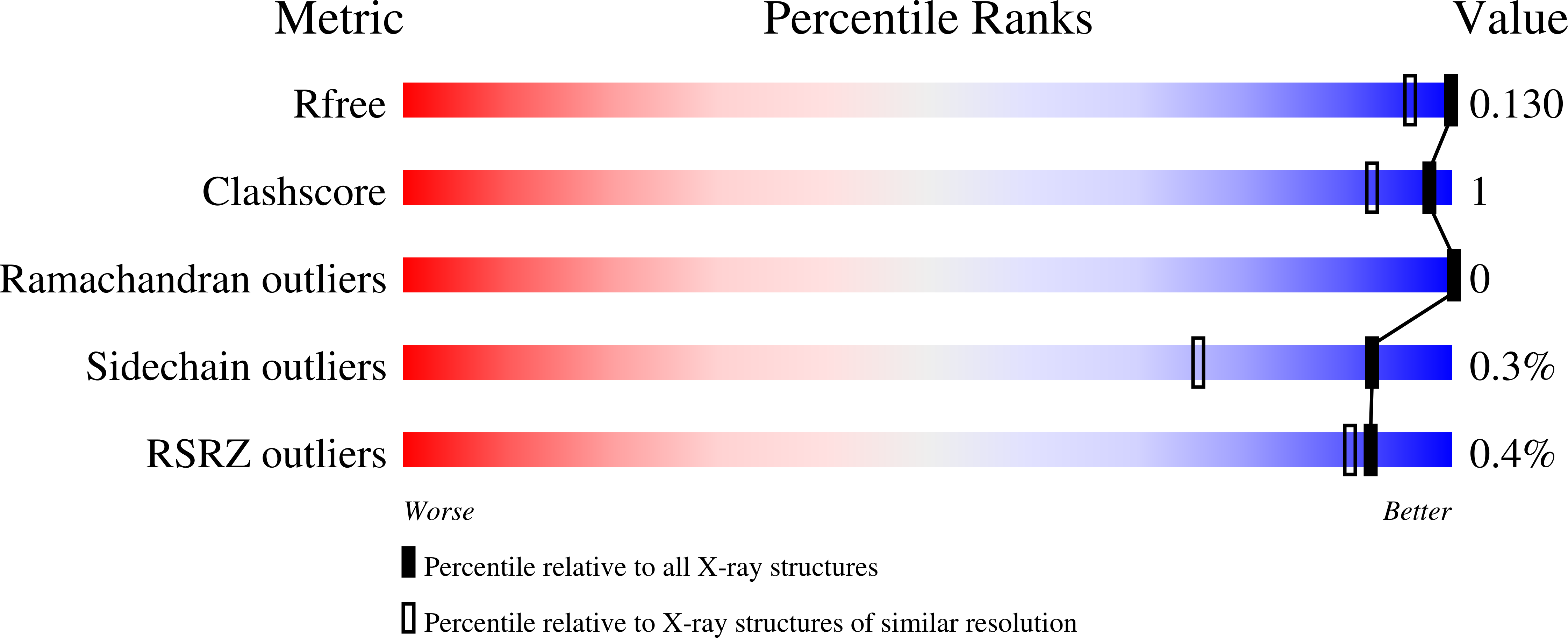
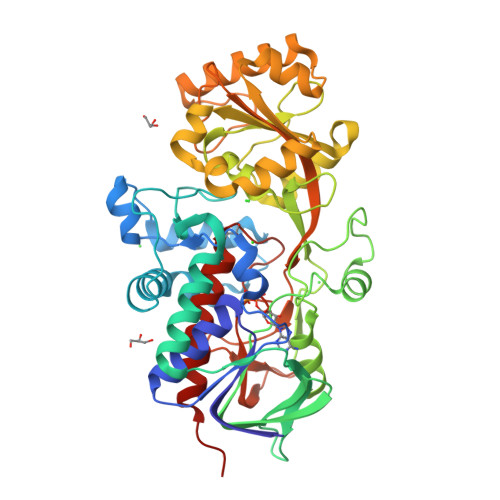
6T85, 6T86, 6T87, 6T88 - PubMed Abstract:
The human microbiome can produce metabolites that modulate insulin signaling. Type 2 diabetes patients have increased circulating concentrations of the microbially produced histidine metabolite, imidazole propionate (ImP) and administration of ImP in mice resulted in impaired glucose tolerance. Interestingly, the fecal microbiota of the patients had increased capacity to produce ImP, which is mediated by the bacterial enzyme urocanate reductase (UrdA). Here, we describe the X-ray structures of the ligand-binding domains of UrdA in four different states, representing the structural transitions along the catalytic reaction pathway of this unexplored enzyme linked to disease in humans. The structures in combination with functional data provide key insights into the mechanism of action of UrdA that open new possibilities for drug development strategies targeting type 2 diabetes.
Organizational Affiliation:
Experimental Medical Science, Medical Structural Biology, BMC C13, Lund University, Lund, Sweden.