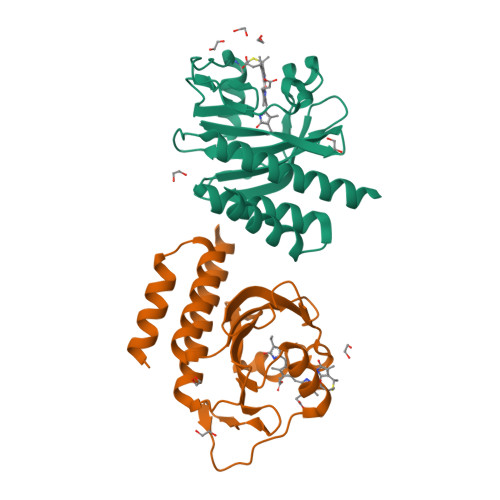
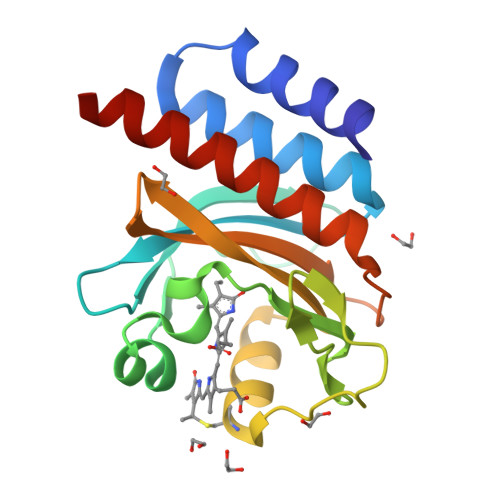
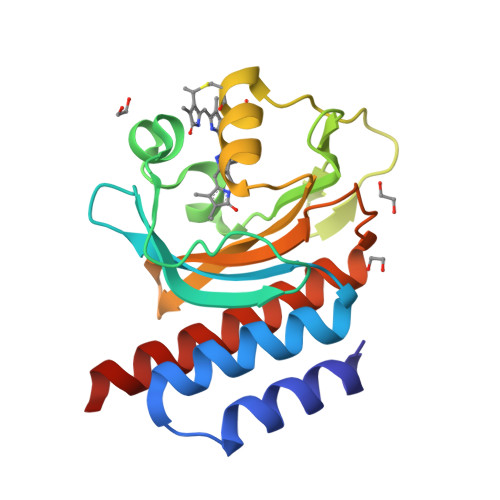
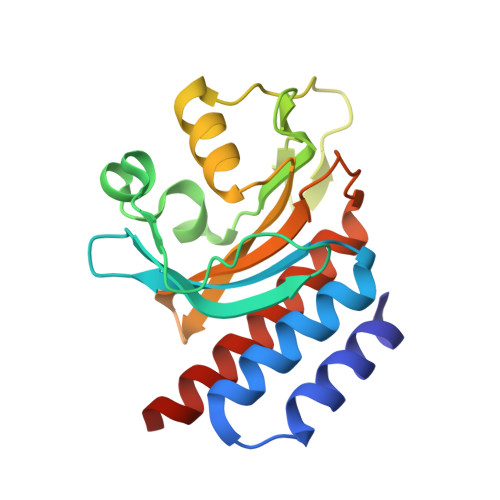
A far-red cyanobacteriochrome lineage specific for verdins.
Moreno, M.V., Rockwell, N.C., Mora, M., Fisher, A.J., Lagarias, J.C.(2020) Proc Natl Acad Sci U S A 117: 27962-27970
- PubMed: 33106421
- DOI: https://doi.org/10.1073/pnas.2016047117
- Primary Citation of Related Structures:
6XHG, 6XHH - PubMed Abstract:
Cyanobacteriochromes (CBCRs) are photoswitchable linear tetrapyrrole (bilin)-based light sensors in the phytochrome superfamily with a broad spectral range from the near UV through the far red (330 to 760 nm). The recent discovery of far-red absorbing CBCRs (frCBCRs) has garnered considerable interest from the optogenetic and imaging communities because of the deep penetrance of far-red light into mammalian tissue and the small size of the CBCR protein scaffold. The present studies were undertaken to determine the structural basis for far-red absorption by JSC1_58120g3, a frCBCR from the thermophilic cyanobacterium Leptolyngbya sp. JSC-1 that is a representative member of a phylogenetically distinct class. Unlike most CBCRs that bind phycocyanobilin (PCB), a phycobilin naturally occurring in cyanobacteria and only a few eukaryotic phototrophs, JSC1_58120g3's far-red absorption arises from incorporation of the PCB biosynthetic intermediate 18 1 ,18 2 -dihydrobiliverdin (18 1 ,18 2 -DHBV) rather than the more reduced and more abundant PCB. JSC1_58120g3 can also yield a far-red-absorbing adduct with the more widespread linear tetrapyrrole biliverdin IXα (BV), thus circumventing the need to coproduce or supplement optogenetic cell lines with PCB. Using high-resolution X-ray crystal structures of 18 1 ,18 2 -DHBV and BV adducts of JSC1_58120g3 along with structure-guided mutagenesis, we have defined residues critical for its verdin-binding preference and far-red absorption. Far-red sensing and verdin incorporation make this frCBCR lineage an attractive template for developing robust optogenetic and imaging reagents for deep tissue applications.
Organizational Affiliation:
Department of Molecular and Cellular Biology, University of California, Davis, CA 95616.