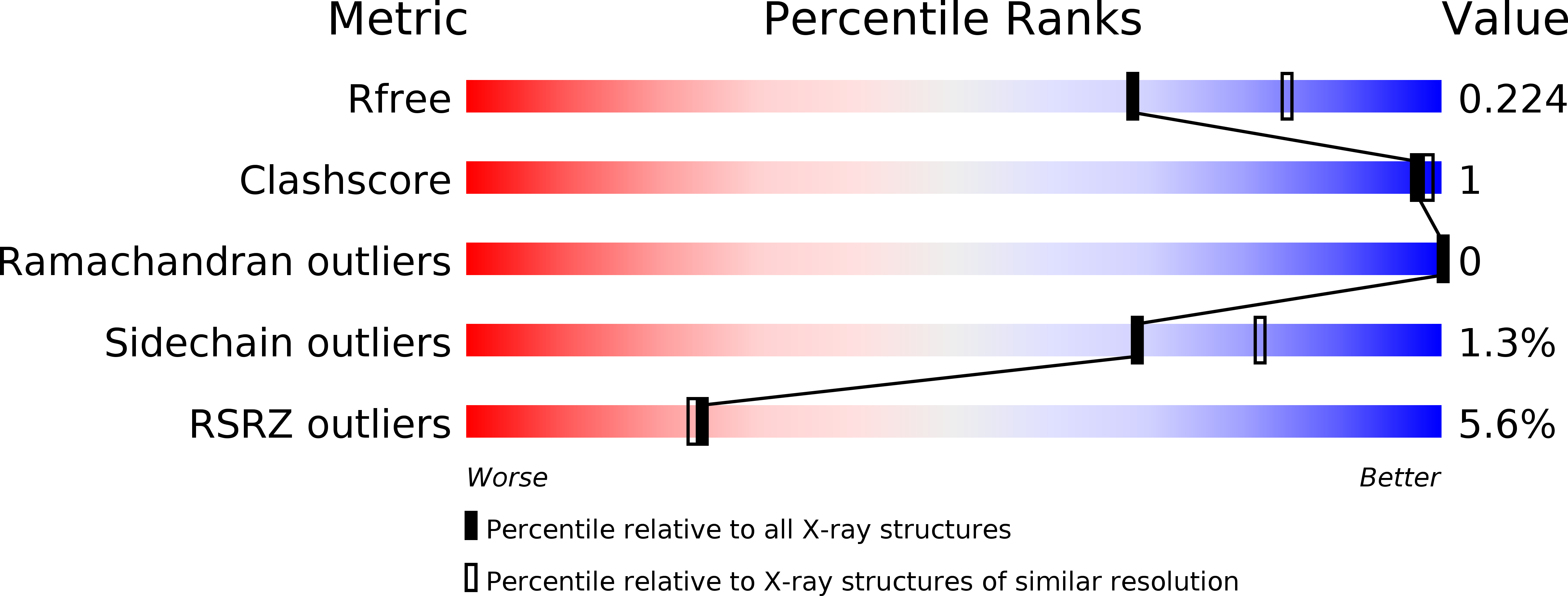
Mapping the Transglycosylation Relevant Sites of Cold-Adapted beta-d-Galactosidase fromArthrobactersp. 32cB.
Rutkiewicz, M., Wanarska, M., Bujacz, A.(2020) Int J Mol Sci 21
- PubMed: 32731412
- DOI: https://doi.org/10.3390/ijms21155354
- Primary Citation of Related Structures:
6ZJP, 6ZJQ, 6ZJR, 6ZJS, 6ZJT, 6ZJU, 6ZJV, 6ZJW, 6ZJX - PubMed Abstract:
β-Galactosidase from Arthrobacter sp. 32cB ( Arth βDG) is a cold-adapted enzyme able to catalyze hydrolysis of β-d-galactosides and transglycosylation reaction, where galactosyl moiety is being transferred onto an acceptor larger than a water molecule. Mutants of Arth βDG: D207A and E517Q were designed to determine the significance of specific residues and to enable formation of complexes with lactulose and sucrose and to shed light onto the structural basis of the transglycosylation reaction. The catalytic assays proved loss of function mutation E517 into glutamine and a significant drop of activity for mutation of D207 into alanine. Solving crystal structures of two new mutants, and new complex structures of previously presented mutant E441Q enables description of introduced changes within active site of enzyme and determining the importance of mutated residues for active site size and character. Furthermore, usage of mutants with diminished and abolished enzymatic activity enabled solving six complex structures with galactose, lactulose or sucrose bounds. As a result, not only the galactose binding sites were mapped on the enzyme's surface but also the mode of lactulose, product of transglycosylation reaction, and binding within the enzyme's active site were determined and the glucopyranose binding site in the distal of active site was discovered. The latter two especially show structural details of transglycosylation, providing valuable information that may be used for engineering of Arth βDG or other analogous galactosidases belonging to GH2 family.
Organizational Affiliation:
Institute of Molecular and Industrial Biotechnology, Faculty of Biotechnology and Food Sciences, Lodz University of Technology, Stefanowskiego 4/10, 90-924 Lodz, Poland.