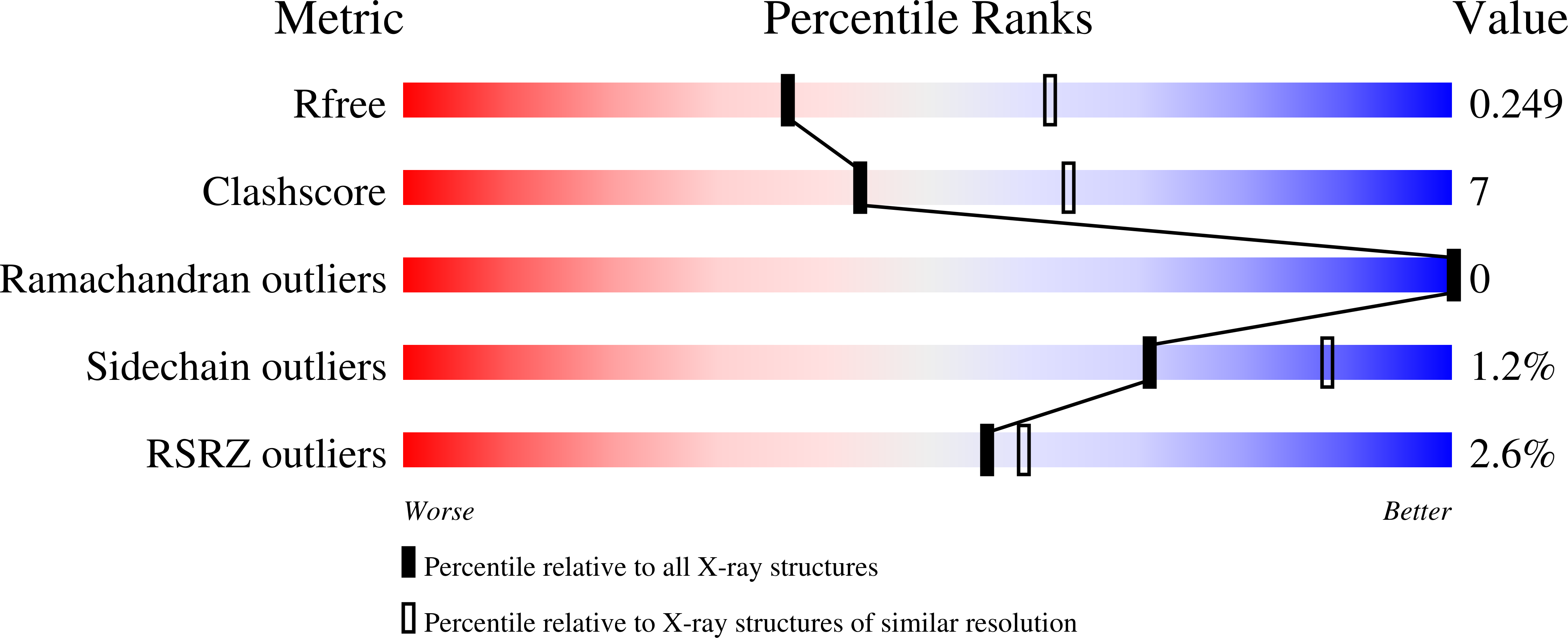
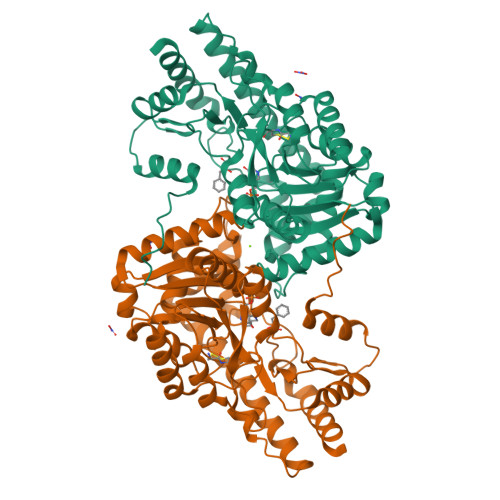
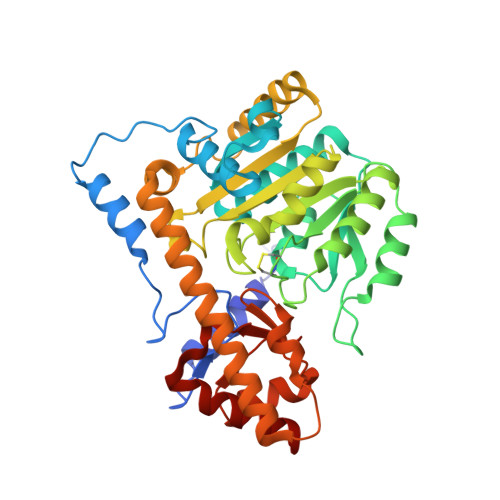
Structural Evidence of Active Site Adaptability towards Different Sized Substrates of Aromatic Amino Acid Aminotransferase from Psychrobacter Sp. B6.
Bujacz, A., Rum, J., Rutkiewicz, M., Pietrzyk-Brzezinska, A.J., Bujacz, G.(2021) Materials (Basel) 14
- PubMed: 34204354
- DOI: https://doi.org/10.3390/ma14123351
- Primary Citation of Related Structures:
6T3V, 6ZUP, 6ZUR, 6ZVG - PubMed Abstract:
Aromatic amino acid aminotransferases present a special potential in the production of drugs and synthons, thanks to their ability to accommodate a wider range of substrates in their active site, in contrast to aliphatic amino acid aminotransferases. The mechanism of active site adjustment toward substrates of psychrophilic aromatic amino acid aminotransferase ( Psy ArAT) from Psychrobacter sp. B6 is discussed based on crystal structures of complexes with four hydroxy-analogs of substrates: phenylalanine, tyrosine, tryptophan and aspartic acid. These competitive inhibitors are bound in the active center of Psy ArAT but do not undergo transamination reaction, which makes them an outstanding tool for examination of the enzyme catalytic center. The use of hydroxy-acids enabled insight into substrate binding by native Psy ArAT, without mutating the catalytic lysine and modifying cofactor interactions. Thus, the binding mode of substrates and the resulting analysis of the volume of the catalytic site is close to a native condition. Observation of these inhibitors' binding allows for explanation of the enzyme's adaptability to process various sizes of substrates and to gain knowledge about its potential biotechnological application. Depending on the character and size of the used inhibitors, the enzyme crystallized in different space groups and showed conformational changes of the active site upon ligand binding.
Organizational Affiliation:
Institute of Molecular and Industrial Biotechnology, Lodz University of Technology, Stefanowskiego 4/10, 90-924 Lodz, Poland.